-
Overview
-
Methods
-
Case Study
-
FAQs
-
Related Resources
-
Related Services
Overview
pKa (Dissociation Constant) is an inherent property of a drug, which is determined by the acidic or alkaline groups within the drug molecule. Generally, the pKa of the drug can be changed by adjusting the acidic or alkaline substructure of the molecule. The degree of ionization of drug molecules in the solution is determined by the pKa of the drug and the pH of the solution. pKa affects the form of drug in body fluids, and pKa is important in drug discovery and development, such as affecting binding to receptors or cell membranes and strategies for salt-type screening. pKa also affects other physicochemical parameters, such as solubility, lipophilicity, dissolution, etc. In addition, pKa can guide preclinical formulation screening and method development of analytical techniques such as chromatography. WuXi AppTec DMPK uses potentiometric and UV spectrophotometric titration method to detect pKa, and mainly uses the Sirius T3 instrument to detect pKa.
Learn More



Methods
-
Test Method
Deion
Potentiometric Titration
Titration range of pH is from 3 to 11;
1-5 mg compound solid powder is requiredUV spectrophotometric Titration
Titration range of pH is from 2 to 12;
20 μL of 10 mM Stock solution is required
Case Study
-
WuXi AppTec DMPK uses Sirus-T3 instrument for the determination of pKa. The potentiometric and UV spectrophotometric titration method applied in the pKa determination are convenient and accurate. PQ data for year 2023 are shown below (The reagents are commercial).
Table 1. PQ data for year 2023
Assay
Dataset No.
Range of acceptable values
Measured value
Date
Propranolol aqueous pKa
23J-23010_Propranolol _pH-metric pKa
9.53 ± 0.3
9.51
23-Oct-2023
Propranolol psKa
23J-23015_Propranolol _pH-metric psKa
9.53 ± 0.3
9.24
23-Oct-2023
Fast UV calibration
23J-24005_Fast UV buffer calibration
N/A
N/A
24-Oct-2023
Hydrochlorothiazide Fast UV pKa
23J-24006_Hydrochlorothiazi_Fast UV pKa
8.75 ± 0.3
9.88 ± 0.38.77,
10.01
24-Oct-2023
Amiloride UV-metric pKa
23J-23020_Amiloride_UV-metric pKa
8.63 ± 0.3
8.66
23-Oct-2023
FAQs
-
What are the principles of potentiometric and UV spectrophotometric titration methods of determination of pKa?
Since ionizable groups change from neutral form to ions form (or conversely) during the titration process, potentiometric titration determines the position and number of pKa based on the change of potential in the titration system, while UV spectrophotometric titration method determines pKa by monitoring the change of UV absorption produced by chromophore group near the ionization center of the compound with pH.
-
What is the difference between the two methods?
Difference
UV spectrophotometric titration method
Potentiometric method
Titration range
pH 2-12
pH 3-11
Compound characteristic requirement
with UV absorption, stable, and high-purity
good solubility, stable, and high purity
Compound requirement
10 mM DMSO stock solution
1 mg of powder
Titration Speed
~25 min/sample
~90 min/sample
-
Will low solubility affect the determination of pKa?
Yes, low solubility can affect the determination of pKa. If the compound shows poor water solubility, it is preferred to use organic co-solvents to improve the solubility.
-
Can we determine the acidity and alkalinity of compound groups by titration? Is that accurate?
It can be determined by the color or slope of the titration-fitted curve in the report, i.e. "acid-red" and "alkali-blue", this is based on the assumption that in a lower dielectric medium, the pKa will shift in favor of the lower dielectric species. This should be the neutral species, but the validity of this statement is unclear, because it does not consider non-specific interactions between the ionized species and the solvent.
Related Resources




-


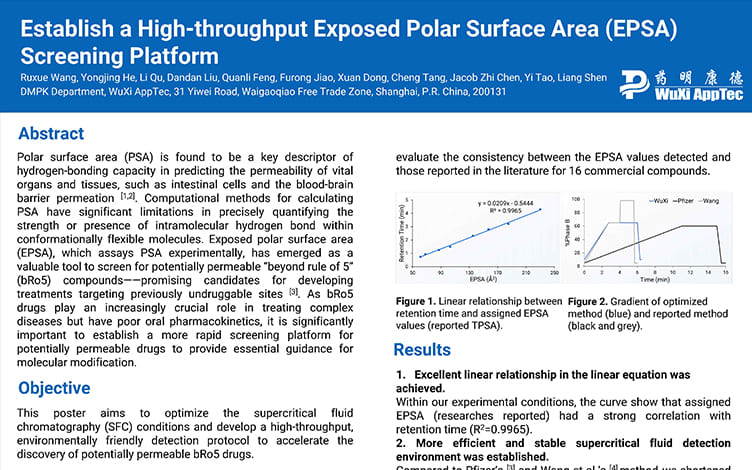
Establish a High-throughput Exposed Polar Surface Area (EPSA) Screening Platform
PostersSep 04, 2025Learn More -


Development of a High-throughput and Cost-effective Method for Experimental Polar Surface Area Measurement with Ultra-Performance Convergence Chromatography Tandem Mass Spectrometry
PostersJul 24, 2025Learn More -


Enhancing Permeability Through Exposed Polar Surface Area (EPSA) for Beyond Rule of Five (bRo5) Drug Candidates
ArticlesApr 10, 2025Learn More -


Rapid Determination of Lipophilicity: Exploration and Establishment of Reversed-Phase Liquid Chromatography (RPLC) Methods
PostersNov 05, 2024Learn More -


Ensuring drug product integrity: The crucial role of stability testing
BlogsOct 27, 2024Learn More -


How to Evaluate Lipophilicity Rapidly? The Significance of Reversed-Phase Liquid Chromatography (RP-HPLC)
BlogsDec 19, 2023Learn More -


Rapid Determination of Lipophilicity: Establishment and Application of Reversed-Phase Liquid Chromatography (RP-HPLC)
ArticlesNov 30, 2023Learn More -


Focusing on PROTAC Permeability and Solubility Improving the Oral Availability
BlogsJul 07, 2023Learn More -


Research on PROTAC Druggability: Solubility and Permeability
ArticlesJun 30, 2023Learn More
References
- 1.
Di L, Kerns E H. Drug-like properties: concepts, structure design and methods from ADME to toxicity optimization[M]. Academic press, 2015.
- 2.
SiriusT3 Instruction Manual
- 3.
《系统药代动力学》杨凌,科学出版社
Stay Connected
Keep up with the latest news and insights.