-
Overview
-
Study Models and Platforms
-
Strengths
-
Study Strategies and Assays
-
Instruments
-
Case study
-
FAQs
-
Related Resources
-
Related Services
Overview
To facilitate the research and development of mRNA-based vaccines and therapeutics, WuXi AppTec DMPK has established an integrated bioanalytical platform. This platform includes qPCR, branched DNA (bDNA), ligand binding assays (LBA), liquid chromatography-mass spectrometry (LC-MS), and flow cytometry, enabling quantitative and semi-quantitative analysis of mRNA. We determine the pharmacokinetic study strategy based on the unique characteristics of each client’s products and provide appropriate study design, accelerating the research and IND application of mRNA-based vaccines, therapeutics, and delivery systems.
Learn More

Study Models and Platforms
-
RT-qPCR quantification analysis
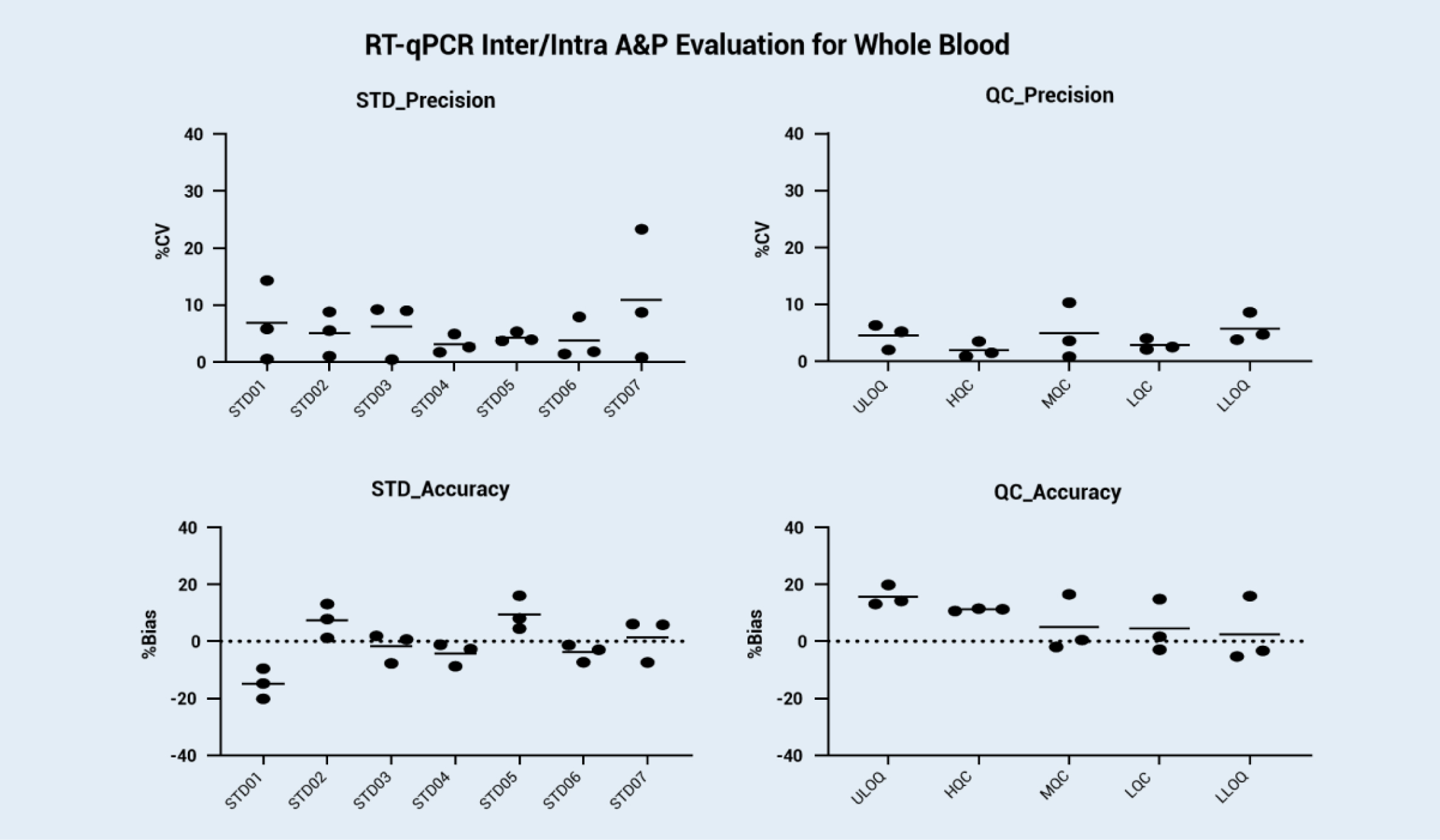
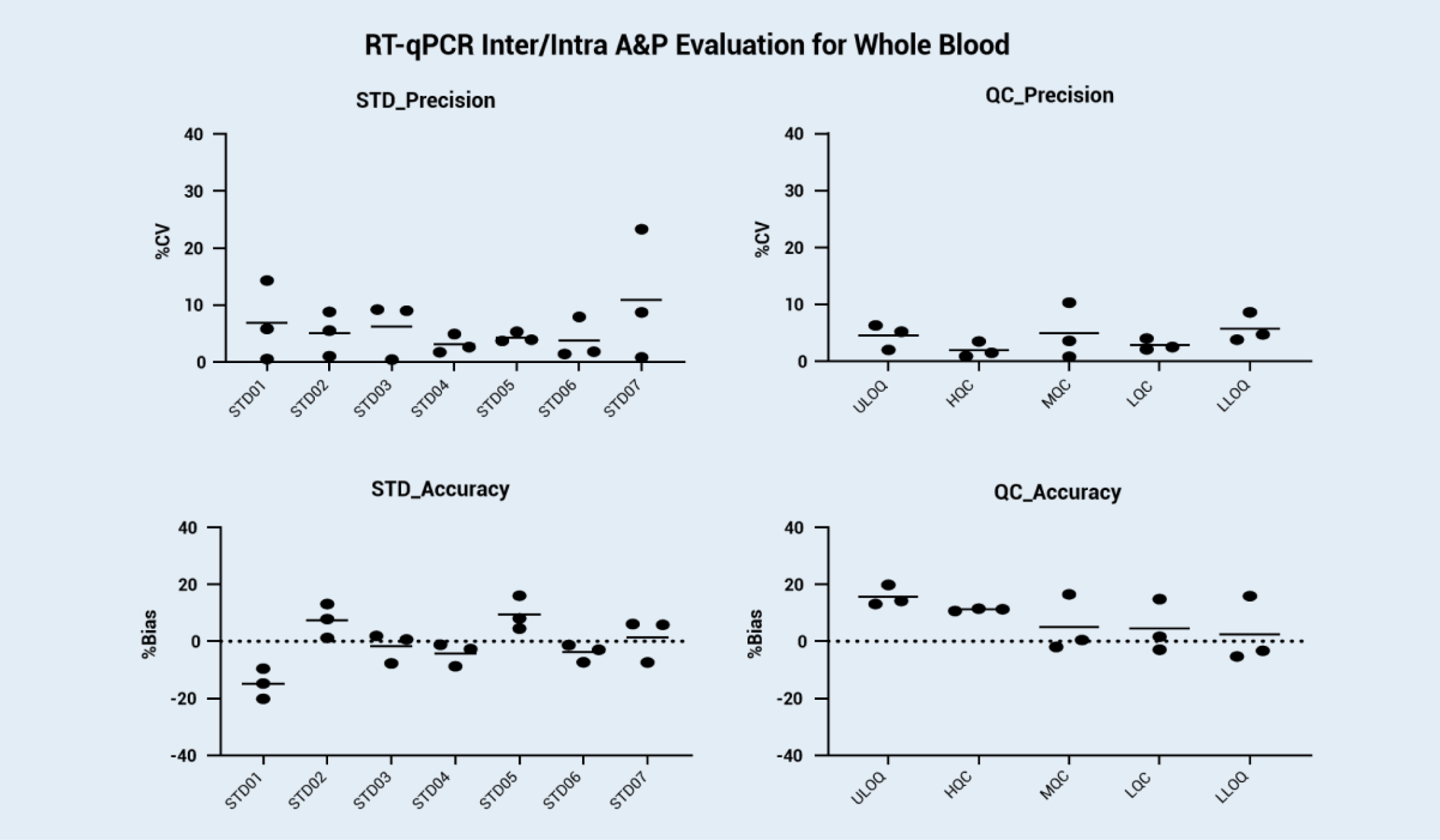
We reference the consensus qPCR validation guidelines on the whitepaper published by the Global CRO Council in Bioanalysis (GCC) and generate our internal mRNA bioanalysis validation guidance. With rich experience in qPCR analysis, we can support fast screening in the development period and IND filing. We developed and validated the mRNA bioanalysis method in rat whole blood and solid tissue (such as liver) by using an mRNA mimic (EGFP mRNA). The standard curve and inter/intra accuracy and precision evaluation from the validation results are shown below.
-
In vivo Fluorescence imaging platform for rodent animals
In vivo Fluorescence imaging platform can mark targets through bioluminescence or fluorescence principles, realize real-time continuous dynamic monitoring of various biological processes in the body, reduce the number of experimental animals used, and reduce the impact of individual differences. It can support the biodistribution and tissue targeting study of delivery system or mRNA.

-
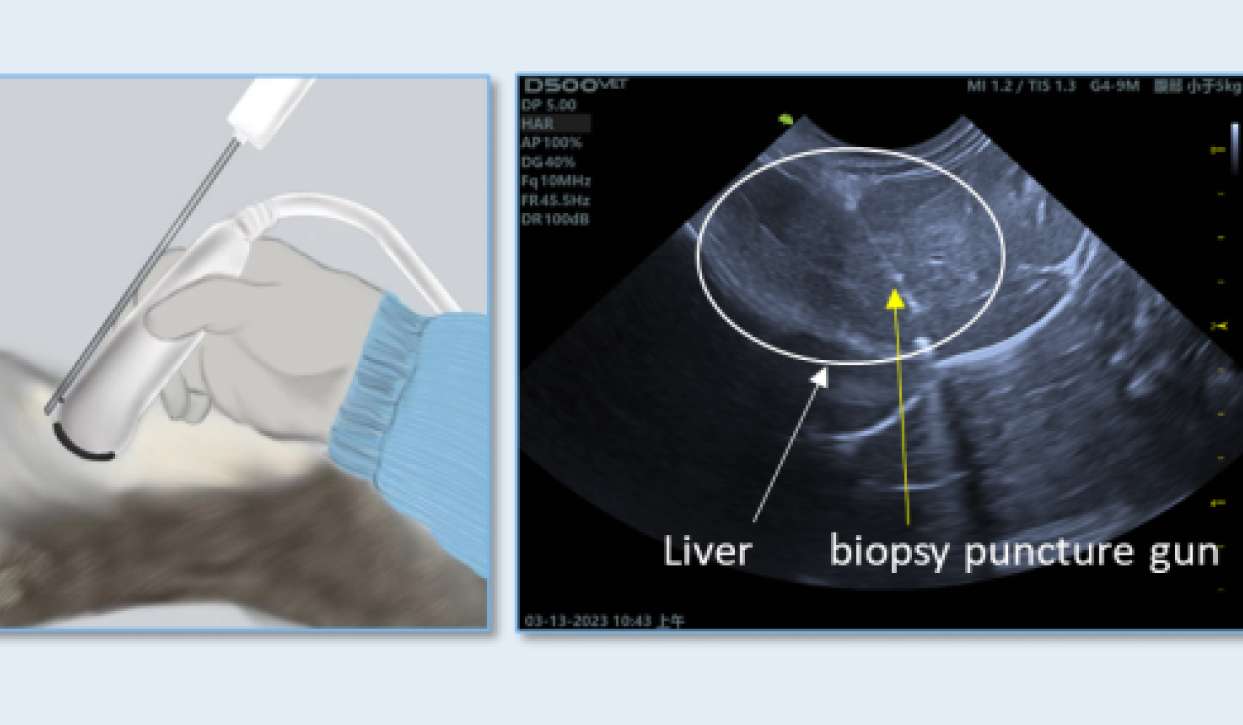
Biopsy technology for large animals
The ultrasound-guided liver biopsy technique in large animals can meet the needs for continuous multiple liver collections, supporting the research of liver-targeted mRNA drugs within large animals.
-
Radioisotope tracer and QWBA platform
The radioisotope tracer and QWBA platform facilitate the investigation of the biodistribution of LNP delivery systems in rodents and cynomolgus monkeys.

Strengths
-
Committed to Your Program
-
Cross-Department Cooperation and High Efficiency
-
Extensive Experience and Customized Research Strategies
-
Comprehensive Capabilities with an Integrated Bioanalytical Platform
Study Strategies and Assays
-
mRNA products comprise two major categories: mRNA vaccines and therapeutics. mRNA vaccines activate the immune system by expressing antigens to achieve preventive or therapeutic purposes, with low dosage and relatively low administration frequency. mRNA therapeutics exert therapeutic effects by expressing functional proteins, while drug efficacy is related to protein expression levels and duration directly. Additionally, the delivery system is an integral component of mRNA products. In preclinical studies, it is necessary to combine the mechanism of action of products and develop appropriate study strategies for different components of the product, to characterize the pharmacokinetic profile comprehensively.
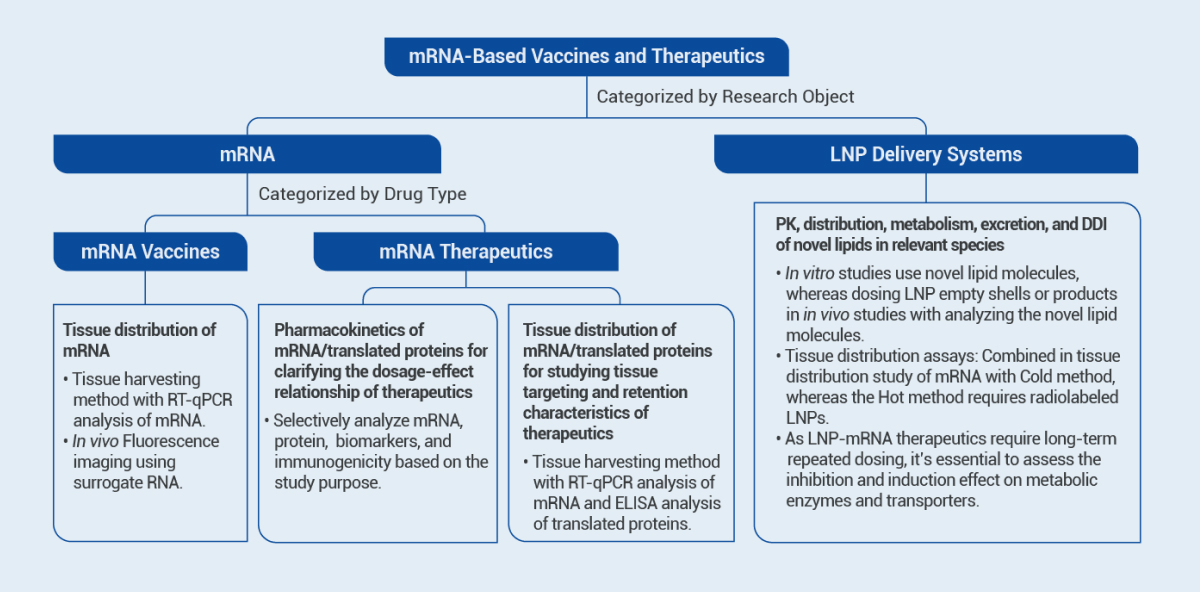
Items | Assays |
mRNA Vaccines | Biodistribution • Tissue distribution study of mRNA |
mRNA Therapeutics | In Vivo PK • Pharmacokinetic study of mRNA/translated proteins • PK/ PD study of mRNA/translated proteins • Immunogenicity study of translated proteins Biodistribution • Tissue distribution study of mRNA/translated proteins |
Lipid Nanoparticle (LNP) Delivery Systems | In Vivo PK • Plasma pharmacokinetic study of LNPs • Immunogenicity of PEG lipids Biodistribution • Tissue distribution study of LNPs In Vitro and In Vivo Metabolism • Stability of novel lipids in plasma, liver microsomes, and hepatocytes • Metabolite identification of novel lipids in liver microsomes and hepatocytes • In vivo metabolite identification of novel lipids Excretion • Excretion study of LNPs Drug Interactions • Inhibition and induction effect on metabolic enzymes by novel lipids • Inhibition effect on transporters by novel lipids |
Instruments
-
-


QuantStudio™ 7 Pro
-


MESO QuickPlex SQ 120
-


Molecular Device M5e
-


BD Fortessa X20
-


Triple Quad 6500 System
-


Orbittrap Eclipse™ Tribrid™
-


Lecia CM3600 XP
-


Amersham Typhoon RGB
-
Case study
-
Background: For IND applications of mRNA-based vaccines and therapeutics, a robust and validated bioanalysis method for mRNA absolute quantification is required. Currently, there is no official guidance for mRNA bioanalysis validations. Here, we reference the consensus qPCR validation guidelines on the whitepaper published by the Global CRO Council in Bioanalysis (GCC) and generate our internal mRNA bioanalysis validation guidance. We developed and validated the mRNA bioanalysis method in rat whole blood and solid tissue (such as liver) by using an mRNA mimic (EGFP mRNA). The validated qPCR method will be suitable for FDA/NMPG/TGA IND application. The standard curve and inter/intra accuracy and precision evaluation from the validation results are shown below.
Learn More
-
FAQs
-
What is mRNA vaccine?
mRNA vaccines involve directly injecting modified mRNA encoding pathogen-specific antigens into the human body after certain processing steps, allowing the body’s protein synthesis system to produce the desired antigens. This type of vaccine does not involve live pathogens in the production or vaccination processes, which eliminates the risk of virus spread and infection. mRNA vaccines can also target the expression of specific antigens, avoiding the adverse effects that other components of pathogens can impose on the human body. Furthermore, it involves “infection” of the cells, which can trigger both humoral and cellular immunity. However, the application of this vaccine technology has a shorter development timeline compared to other vaccine technologies and thus requires extensive validation.
-
What is the difference between mRNA vaccines and mRNA-based therapeutics?
First, mRNA vaccines encode antigenic proteins and generally require only a low level of protein expression to exert their effects. The induction of antigenicity in the body reflects their efficacy. In contrast, mRNA therapeutic drugs generally require a high level of protein expression. And it is desirable to minimize the stimulating immunogenicity, as that may reduce the concentration of the therapeutic protein. Second, vaccines do not require tissue-specific delivery, but mRNA therapeutic drugs must be delivered to specific tissues for translation. Third, most vaccines are injected into muscles because muscle tissue has extensive blood flow and high levels of infiltrating immune cells. Whereas, mRNA therapeutic drugs are mostly administered systemically or topically to target tissues according to their therapeutic purpose. Overall, mRNA vaccine development technology has been well established, whereas the development of mRNA therapeutic drugs has faced several challenges, including improving protein expression levels, reducing immunogenicity, improving tissue targeting, exerting long-term effects, and improving long-term safety for repeated use.
Related Resources




-


mRNA Therapeutics Quantification: RT-qPCR vs. bDNA Analytical Methods
ArticlesJan 23, 2026Learn More -


Liposome Drug Delivery: Classification, Composition, and Formulation Considerations
ArticlesJan 16, 2026Learn More -


mRNA Vaccines and Therapeutics: Advances in Modification, Delivery, Applications, and Analytical Strategies
ArticlesJul 17, 2025Learn More -


An Efficient Bioanalytical Method for mRNA Drug in Mouse Blood and Tissue Samples
PostersApr 17, 2025Learn More -


Comparison of Branched DNA (B-DNA) and Polymerase Chain Reaction (RT-qPCR) Method Validation for the Quantification of mRNA Therapeutics in Plasma
PostersJan 12, 2024Learn More -


mRNA-Based Vaccines and Therapeutics DMPK Services
BrochuresJan 04, 2024Learn More -


What Can We Learn from the PK Profiles of FDA Approved mRNA Vaccines
BlogsDec 29, 2023Learn More -


Vaccine Development Driven by SARS-CoV-2: The Promising Prospect of mRNA Vaccines
BlogsDec 19, 2023Learn More -


FDA Approved mRNA Vaccines: Interpretation of Preclinical Pharmacokinetic (PK) Data
ArticlesDec 19, 2023Learn More -


How to Conduct Preclinical Pharmacokinetic Evaluation of mRNA-Based Therapeutics?
BlogsDec 15, 2023Learn More -


What is mRNA Vaccines and Its Future?
ArticlesDec 08, 2023Learn More -


Strategies for Preclinical PK Evaluation of mRNA-Based Therapeutics: mRNA Vaccines, mRNA Therapeutic Drugs, and Novel Excipients
ArticlesDec 08, 2023Learn More -


Evaluation of mRNA Capping Efficiency by Liquid Chromatography Coupled to High Resolution and Accurate Mass Spectrometry
PostersJun 01, 2023Learn More
Stay Connected
Keep up with the latest news and insights.