-
Overview
-
Study Models and Platforms
-
Experience
-
Study Strategies and Assays
-
Instruments
-
Case Study
-
FAQs
-
Related Resources
-
Related Services
Therapeutic Areas
Overview
Respiratory diseases have been the third most common chronic diseases after cardiovascular diseases and diabetes. The respiratory drugs include antitussive, expectorant, and antiasthmatic. The forms of drugs include injection drugs and oral drugs, as well as inhaled drugs which could be distributed to the target site directly. With the continuous development of medical and pharmaceutical, as well as our deepening understanding of pulmonary function and diseases like asthma and chronic obstructive pulmonary disease (COPD), inhaled delivery has been recognized as the optimal and most effective administration route for treating respiratory diseases. According to market statistics, the sales of inhaled drugs, including aerosol, dry powder, and sprays, have consistently risen over the past decade. As such, inhaled drug delivery is attracting more and more attention in the pharmaceutical industry. Since 2013, WuXi AppTec DMPK has conducted respiratory disease research, gathering rich R&D experience. In addition to the routine administration routes, we have mastered the challenging inhaled delivery route, and have completed over one hundred inhaled drug projects, successfully advancing many respiratory drugs to the clinical stage.
Learn More

Study Models and Platforms
-
Devices for inhalation administration
The platform can provide devices for nose-only exposure inhalation administration, intranasal administration, and intratracheal atomization administration.

-
Physicochemical property testing and formulation screening
In the early stage of drug development, the physicochemical properties are crucial for the formulation screening. The WuXi AppTec DMPK has a robust physicochemical property testing platform and extensive experience in formulation screening.

-
Bioanalysis
The WuXi AppTec DMPK has extensive experience in biological sample analysis and is equipped with high-precision analytical instruments to achieve the lower limit of quantitative analysis for various samples. WuXi AppTec DMPK has a mature ligand-binding assay and liquid chromatography-mass spectrometry (LC-MS/MS) analysis platform to enable the quantification of large- and small-molecule drugs in samples. The platform can simultaneously detect urea concentration (correction factor) in plasma/serum and alveolar lavage fluid samples. Pharmaceutical analysis of drugs, such as inhaled monoclonal antibodies, is arduous and requires high sensitivity. However, the detection limit of the enzyme-linked immunosorbent assay/Meso Scale Discovery (MSD) method we developed is as low as 1 ng/mL. We are also equipped with LC-MS/MS and MSD devices for the detection of biomarkers related to lung diseases, lysophosphatidic acid (LPA16:0, 18:0, 18:1, 18:2, 20:4), and cytokines.

Experience
-
10+
Years of experience
-
100+
Inhaled delivery projects
Study Strategies and Assays
Whether the drug is administered via systemic or inhaled routes, comprehensive pharmacokinetic and pharmacodynamic studies should be conducted in early non-clinical studies to establish good pharmacokinetic (PK) / pharmacodynamic (PD) correlations.
For respiratory drugs that function through systemic administration, the study strategy references the study of conventional systemic administration of the drug to conduct in vitro ADME, drug-drug interaction, and in vivo PK studies and special attention should be paid to the distribution of the test articles in lung tissue.
Since respiratory drugs administered via inhalation can also enter the system transpulmonary absorption or after being swallowed, the development strategy refers to the systemic drug development strategy and includes the stability test in lungs. In the early stage of drug development, the solubility of the drug affects the preparation of the formulation, thus, an investigation of the physicochemical properties is recommended to provide a reference for formulation screening.
| Assay type | Discovery | IND Filling | |||
Systemic administration | Inhalation administration | Systemic administration | Inhalation administration | ||
In vitro | Caco-2 cell permeability assay |  | Option* |  | Option* |
In vitro plasma protein binding assay |  |  |  |  | |
Metabolic stability test (liver microsomes and hepatocytes) |  |  |  |  | |
Metabolic stability test (lung microsomes/lung S9/ lung homogenate) |  | Option* | |||
Metabolite identification test liver microsomes and hepatocytes) |  |  |  |  | |
Metabolizing enzyme phenotyping assay |  |  | |||
Drug-drug interaction assay |  |  |  |  | |
Physicochemical properties assay |  |  | |||
In vivo | Bioavailability |  |  (with additional oral bioavailability studies) |  |  |
Plasma pharmacokinetic studies of intravenous and extravascular administration in rodent and non-rodent animal models |  |  |  |  | |
Tissue distribution (with special attention to lung tissue distribution) |  |  |  |  | |
Measurement of drug concentration in alveolar lavage fluid (concentration in lung epithelial lining fluid) | Option* | Option* | |||
Biliary, fecal, and urinary excretion |  |  | |||
In vivo metabolite identification test |  |  | |||
QWBA | Option* | Option* | |||
*Determined by the actual situation.
Instruments
-
Device for inhalation administration
-
Physicochemical property testing and formulation screening
-
Bioanalysis
-
-


Mainframe and atomizing exposure
-


Solution atomizer
-


Dry powder generator
-


Filter (machine)
-


Photometers
-


Cascade
-

Intranasal instillation
-


Intranasal nebulizer
-

MicroSprayer Aerosolizer
-


DPI specific Syringe
-
-
-


NEPHEL Ostar Plus Nephelometer
-


Sirius T3
-


Covaris® E200x Ultrasonic Dispersers
-


Malvern® Mastersizer 3000 Laser Diffraction
-

Osmolality Pro
-
-
-


Triple Quad 6500 System
-


Molecular Devices SpectraMax M5e
-
Case Study
-
Taking propranolol, a small-molecule drug, as an example, the bioavailability of conventional oral administration (PO), intratracheal nebulization administration (IT), and nose-only exposure inhalation administration was investigated.
Experimental design: Twelve mice were randomly divided into four groups and given propranolol via intravenous injection, oral administration, IT administration, and nose-only exposure inhalation. After dosing, plasma samples were collected for the drug concentration.
The pharmacokinetic curves after dosing via each route are shown as below, and the PK parameters are shown in the Table. Due to the extensive hepatic first-pass effect, the bioavailability of propranolol after oral administration was low, however, the bioavailability increased significantly after IT administration and nose-only exposure inhalation. The results of this study provided data to support altering the administration route, reducing the treatment dose, and improving the bioavailability of drugs with low oral bioavailability, especially for pulmonary diseases.
Learn More
System exposure after propranolol hydrochloride administration via different routes
Dosing route
Dose level (mg/kg)
C0/Cmax (ng/mL)
AUC0-inf (ng.h/mL)
Bioavailability
IV
5
832
1341
-
PO
10
90.7
135
5.05%
IT
2.2
907
633
107%
Nose-only exposure Inhaltion
2.04
210
286
52.2%
-

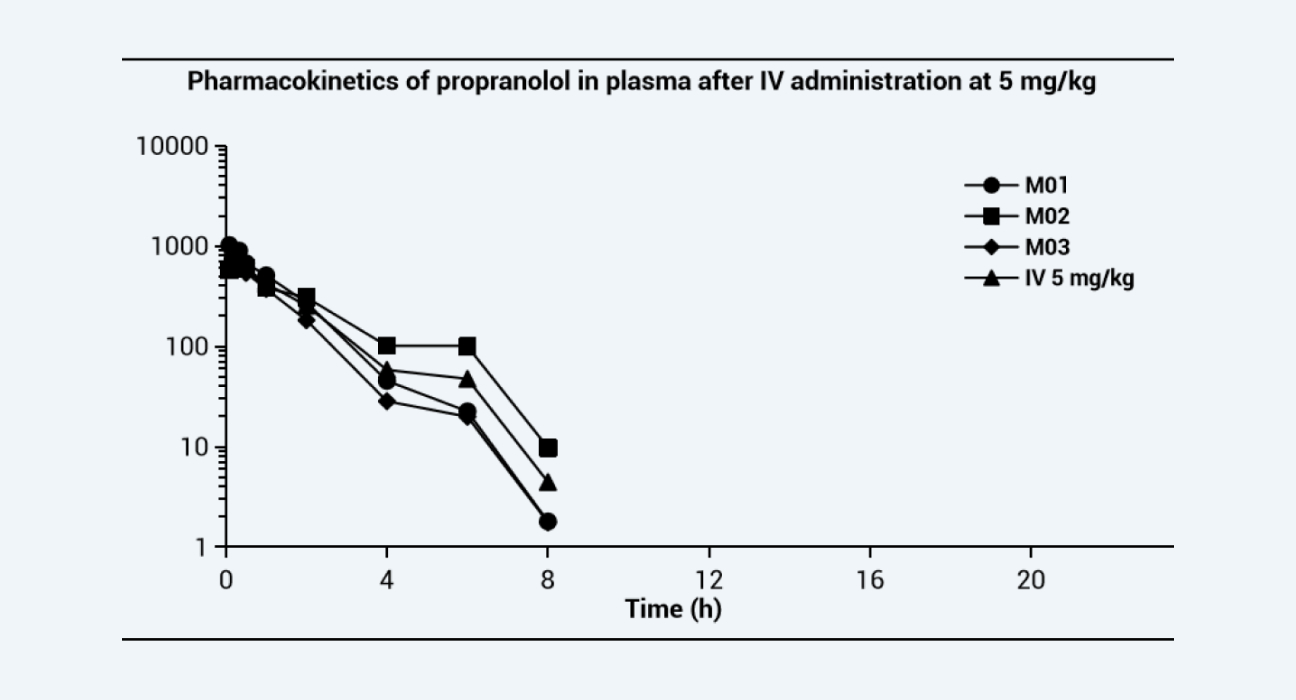
Pharmacokinetics of propranolol in plasma after IV administration at 5 mg/kg
Figure 1
-

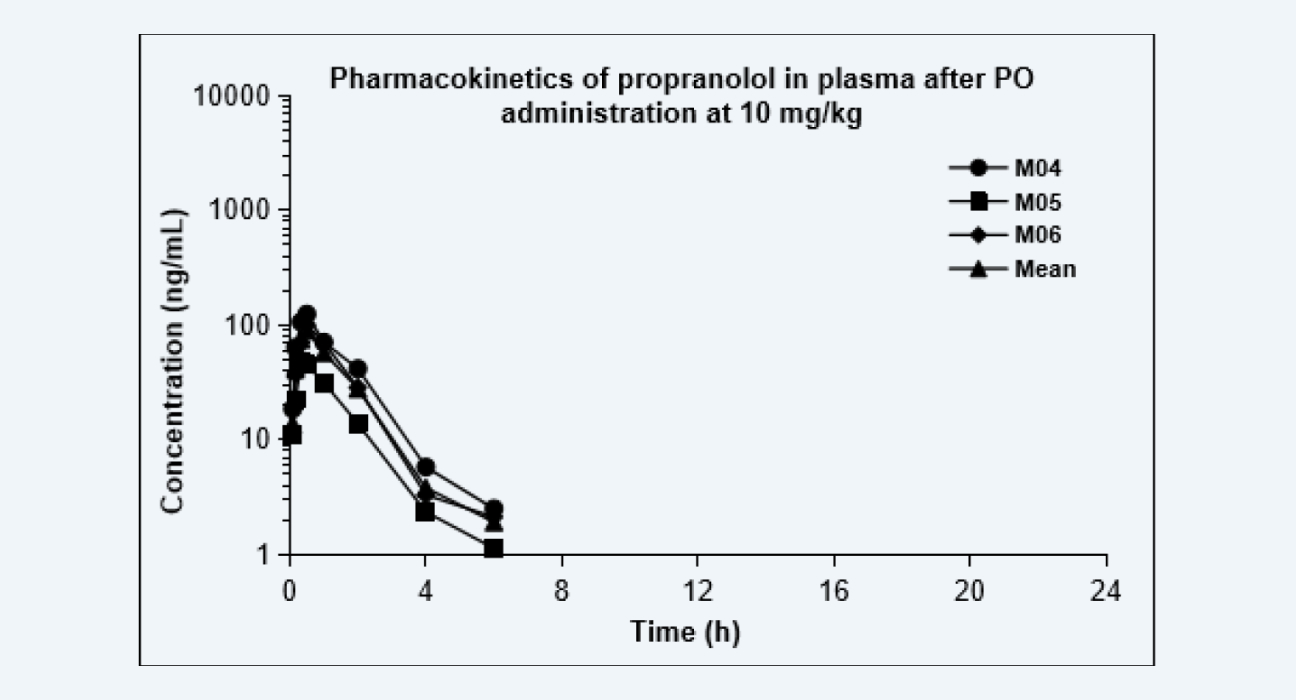
Pharmacokinetics of propranolol in plasma after PO administration at 10 mg/kg
Figure 2
-

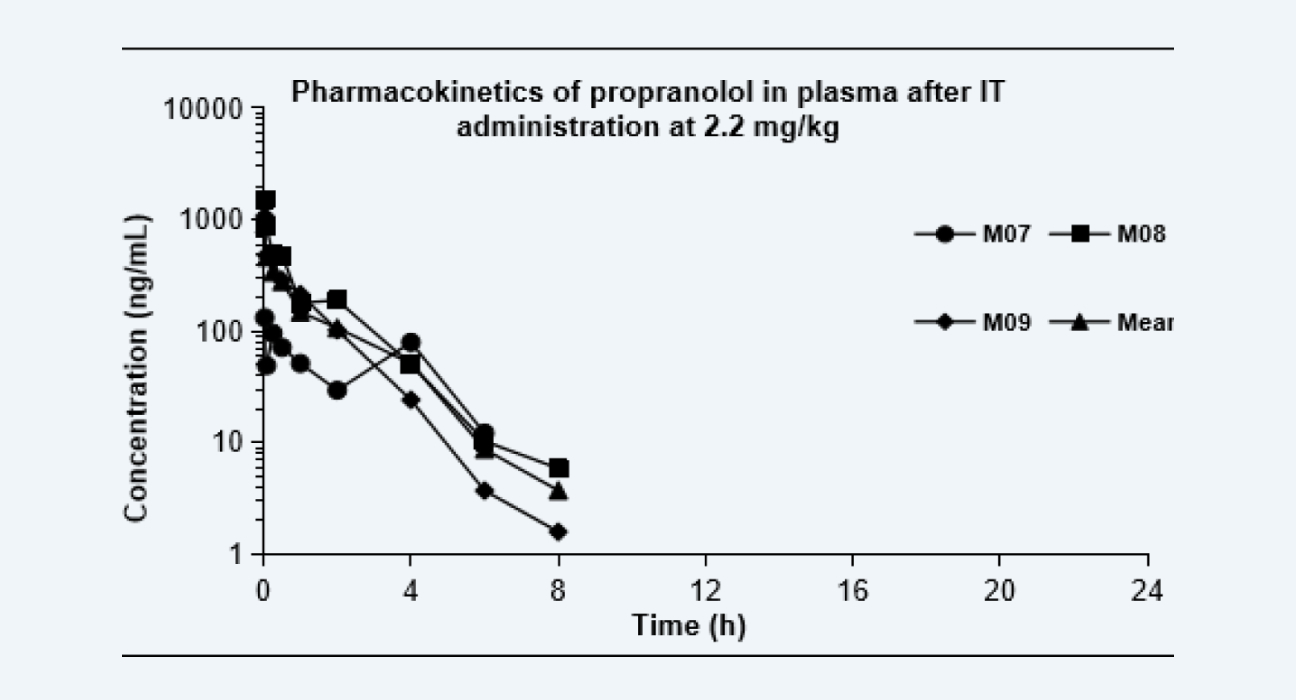
Pharmacokinetics of propranolol in plasma after IT administration at 2.2 mg/kg
Figure 3
-

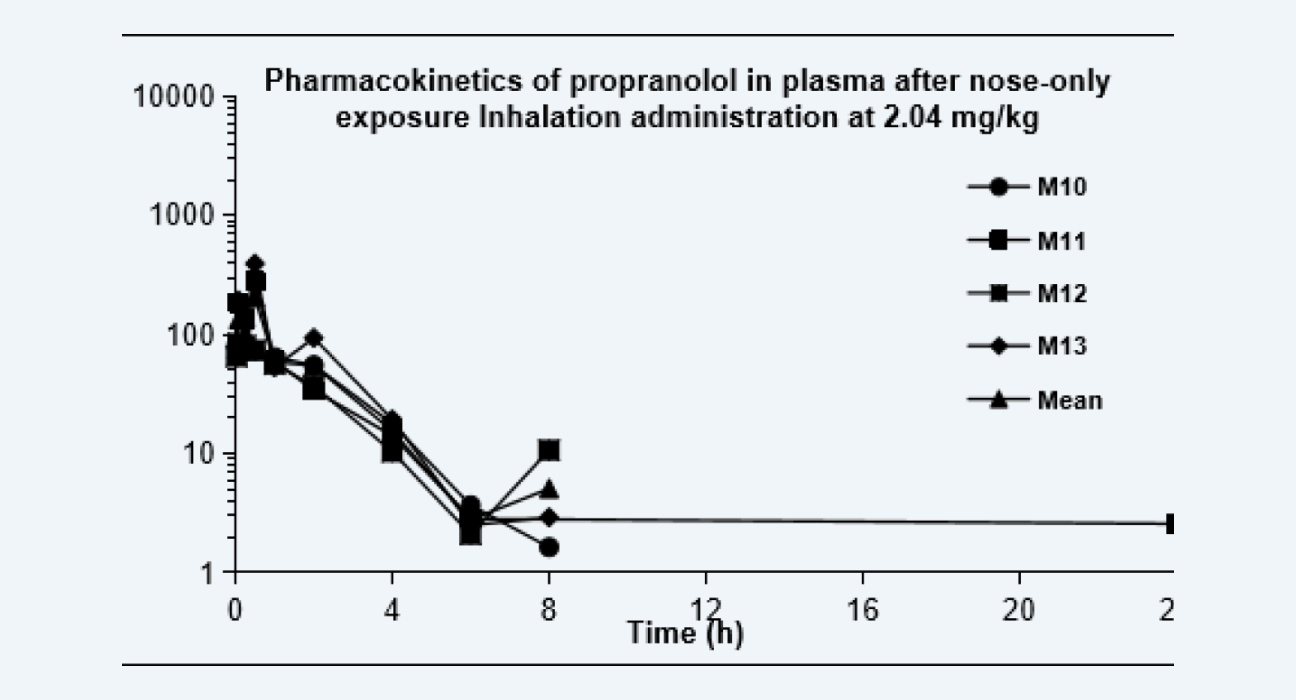
Pharmacokinetics of propranolol in plasma after nose-only exposure Inhalation administration at 2.04 mg/kg
Figure 4
-

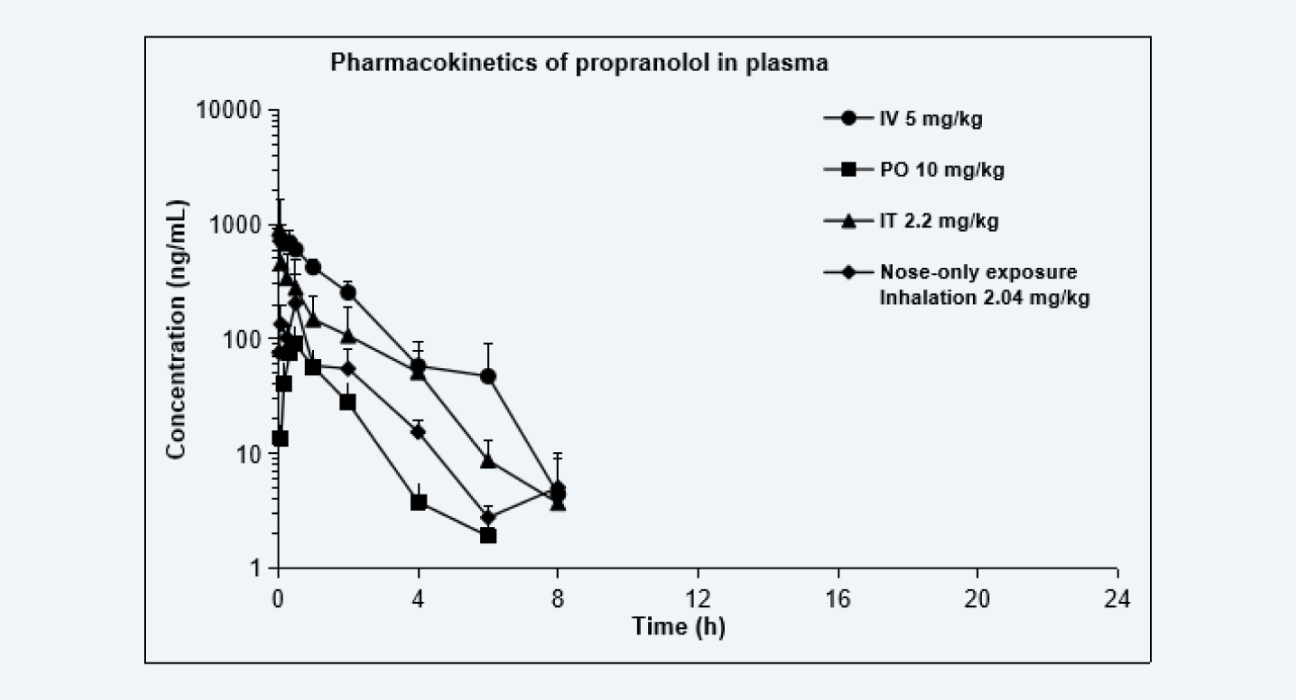
Pharmacokinetics of propranolol in plasma
Figure 5
-
FAQs
-
How do respiratory drugs work?
Respiratory drugs include antitussive, expectorant, and antiasthmatic. Antitussive drugs are divided into central antitussive drugs and peripheral antitussive drugs. The central antitussive drugs target the cough center of the brain and the peripheral antitussive drugs inhibit the receptors in the cough reflex and the endings of the afferent nerve fibers. Expectorant drugs reduce the viscosity of sputum by dissociating mucin fibers, mucin molecules, DNA in sputum, etc. to make sputum easy to be discharged, or increase the mucociliary movement, and improve the mucus transport function. The pharmacological effects of antiasthmatics include reducing the release of inflammatory mediators, relaxing airway smooth muscle, and stabilizing hypertrophic cell membranes.
-
How are respiratory drugs administered?
The dose routes of respiratory drugs include regular injection, oral administration, intranasal administration, intratracheal administration, and nose-only exposure inhalation administration, etc.
-
How to select the delivery devices for inhaled drugs?
The choice of drug delivery device should be based on various factors, including the drug development stage, formulation type, and quantity of the compound. In the early stages of drug development, only limited quantities of the compound are available, and intranasal or intratracheal administration can be adopted to study the pulmonary PK properties using a small amount of the compound. If the compound amount is large, the nose-only exposure inhalation tower can be used for drug research.
-
What are the requirements of inhalation formulation?
Based on the research stage and research purpose, the compound powder or the prepared formulation could be selected for inhalation delivery research. During the early stages of drug development, when the properties of the compound are not yet determined, it is advisable to attempt formulating the compound into a clear solution for administration. Once the characterization of the target formulation has been established and the manufacturing process is mature, it is possible to directly provide inhaled formulations for administration.
Related Resources




-


Respiratory Medications DMPK Services
BrochuresDec 25, 2024Learn More -


Unleashing the Potential of Inhaled Drugs: Challenges and Strategies for Preclinical In Vivo PK Studies
BlogsFeb 27, 2024Learn More -


Illuminating the Path of Treating Chronic Obstructive Pulmonary Disease (COPD): Exploring the PK Profiles of Approved Inhaled Medications
BlogsJan 23, 2024Learn More -


Inhaled Medications: Challenges and Strategies for Preclinical In Vivo PK Studies
ArticlesJan 12, 2024Learn More -


Treating Chronic Obstructive Pulmonary Disease (COPD): Analysis of PK Profiles of Approved Inhaled Medications
ArticlesDec 29, 2023Learn More
Stay Connected
Keep up with the latest news and insights.