-
Overview
-
Study Model and Platform
-
Experience
-
Study Strategies and Assays
-
Instruments
-
Case Study
-
FAQs
-
Related Resources
-
Related Services
Therapeutic Areas
Overview
Compared to the other types of drug development, ophthalmic drug research exhibits characteristics of multiple biological barriers, significant analytical challenges, elaborate experimental operations, and lack of guidelines, posing challenges to R&D institutions.
Since 2014, WuXi AppTec DMPK has conducted ophthalmology research, gaining experience and expertise in preclinical ophthalmic drug development of small molecular and novel molecular entities, including peptides, antibodies, oligos, etc. We have mastered multiple routes of administration for the anterior and posterior segments of the eye, including eye drop instillation, intracameral injection, subconjunctival and retrobulbar injections, etc. We demonstrate this expertise by performing high-quality dissection, precise sample processing, and bioanalysis of various ocular tissues. With comprehensive internal capabilities, we can collaborate with other teams within the WuXi AppTec platform to offer customers integrated R&D services.
Learn More


Study Model and Platform
-
In vitro platform
WuXi AppTec DMPK has established high-throughput in vitro models for drug–melanin binding, and the ophthalmic permeability assays, including completed cornea and scleral permeability assays, which are fast, efficient, and cost-effective. These models facilitate a more precise understanding of the permeability, distribution, and accumulation of topical ophthalmic drugs, providing a valuable reference for relevant pharmacokinetics and pharmacological studies.

-
Ocular drug delivery
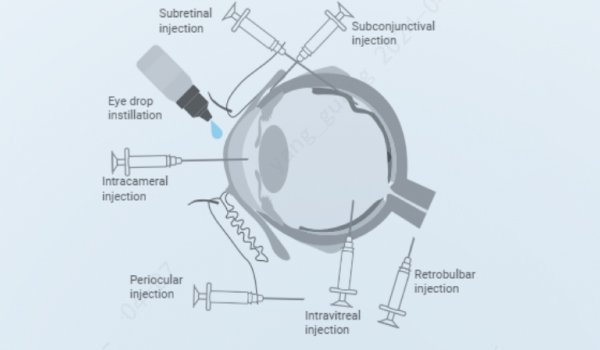
The platform offers a wide range of ocular administration routes for various animal species (e.g., mice, rats, rabbits, dogs and monkeys), including eye drop, ointments or gel application, intracameral injection, subconjunctival injection, intravitreal injection, subretinal injection, pericular injection and retrobulbar injection.
-
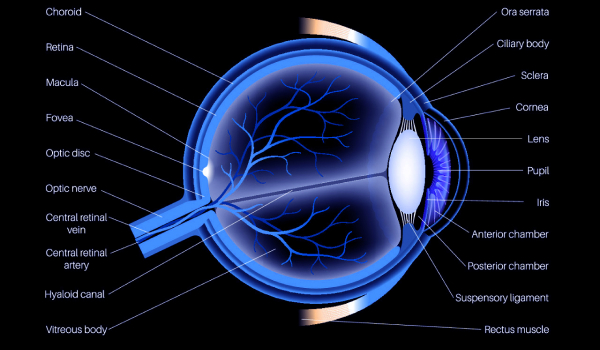
Sample collection
Type of
sampleConjunct
ivaCornea Iris Ciliary
bodyCrystalline
lenRetina Choroid Sclera Vitreous
humorLacrimal
glandLower
eyelidOptic nerve Aqueous
humorTear Mouse
Rat
Rabbit
Dog
Monkey
-
Ophthalmic sample processing
Ocular tissues vary in size, structure, and characteristics, necessitating different processing methods. The platform provides different homogenization ratios and homogenization using different types of equipments, such as high-shear homogenizers, ball mills, and multifunctional biological sample homogenizers, and ensures the tissue homogenation is completed and uniformal.

-
Ophthalmic examination
WuXi AppTec DMPK has professional veterinary team, comprehensive ophthalmic examination equipments, and well-established ocular scoring standards (Draize Eye Irritation scoring system and McDonald-Shadduck scoring system). These platforms provide various ophthalmic examinations, including intraocular pressure detection, pupillary reflex examination, slit lamp examination, fundus imaging, and sodium fluorescein staining.
-
Ocular sample analysis
WuXi AppTec DMPK has extensive experience in the anlysis of ocular biological for both large and small molecular. Equipped with a wide range of highly sensitive and precise bioanalytical instruments, we analyzed over 5000 ocular samples annually, with a quantification limit as low as 5 pg/ml. We have innvovated unique methodologies to address challenges encountered in the bioanalysis of ocular samples, such as low drug concentrations in the systemic circulation, small sample volumes, difficulties in tissue homogenization, quantification of samples collected using special collection methods challenges in obtaining blank matrices, and the specific binding of drug to melanin. These methodologies ensure the provision of accurate and reliable analytical data.

Experience
As part of radiolabeled ADME Network, WuXi AppTec DMPK offers a full range of 14C & 3H custom radiolabeling services. With more than 10 years experiences, our team of synthetic radiochemists can prepare varieties of radiolabeled compounds, including small molecules, ADC, PDC, Proteolysis-Targeting Chimeras (PROTACs*), Peptide, etc.
-
10+
Years of experience
-
150+
Ophthalmic projects
Study Strategies and Assays
For ophthalmic drugs that function through systemic administration, the R&D strategy should focus on the tissue distribution of the compound in the ocular region, in addition to the reference to conventional systemic drug delivery studies.
For ophthalmic drugs that exert drug efficacy through ocular topical drug delivery: If a certain amount of the compound enters the systemic circulation after ocular topical administration, the R&D strategies should refer to the drugs into systemic administration. However, if no or minimal amount of compounds enters the systemic circulation after ocular topical administration, the research should focus on the distribution of the compound in ocular tissues.
In vitro melanin binding assays are useful for guiding the selection of animal species for ophthalmic drug studies and are recommended to be evaluated using the appropriate animal models in the early stages of preclinical development.
| Research projects | Ocular topical route of administration | Systemic route of administration | |||
|---|---|---|---|---|---|
In vitro melanin binding assay | Required | On a case-by-case basis | |||
In vitro plasma protein binding assay | Required if it enters the systemic circulation | Required | |||
In vitro metabolic stability assay | |||||
In vitro drug-drug interaction assays | |||||
In vitro corneal/scleral permeation assay | Selection on a case-by-case basis | ||||
In vivo absorption | Species selection | Two species are recommended, large molecular selection of pharmacodynamically relevant species | Two species are recommended, large molecular selection of pharmacodynamically relevant species | ||
Number of samples | 3 samples/sex/time point | 3 samples/sex/time point | |||
Number of timepoints | ≥ 5 | Small molecules: 10–12; large molecules: 13–15 | |||
Route of administration | Ocular topical administration | IV, PO, SC, IM, etc. | |||
Frequency of administration | Single or multiple | Single or multiple | |||
In vivo distribution | Ocular tissue distribution required | Required | |||
In vivo excretion | On a case-by-case basis | Required | |||
In vivo metabolite identification | |||||
Instruments
WuXi AppTec DMPK is equipped with various high-precision equipment and instruments, which can be efficient and produce high-quality study results.
-
-


Intraocular Pressure Meter
-


Slit Lamp
-


Lenses
-


Indirect Inspection Glasses
-


Ophthalmic Microscope
-


Shear Homogenizer
-


Ball Mill
-


Multifunctional Boilogical Sample Homogenizer
-


Sciex Triple Quad™ 7500
-


Molecular Devices SpectraMax M5e
-


MESO QuickPlex SQ 120
-
Case Study
-
-

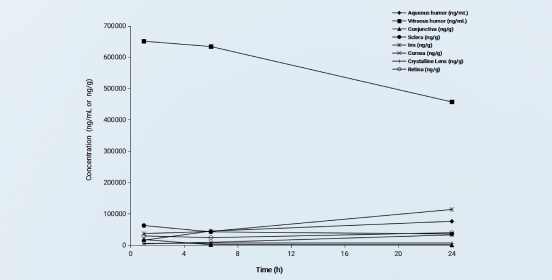
Average tissue drug concentrations after a single intravitreal injection of commercialized vancomycin in New Zealand rabbits
Figure 1
Taking intravitreal injection of commercialized vancomycin in New Zealand rabbits for an example, after a single intravitreal injection of vancomycin at 1.00 mg/eye, aqueous humor, vitreous humor, sclera, iris, cornea, conjunctiva, retina, lens, and plasma were collected to evaluate the distribution of the drug in ocular tissues.
To provide high-quality samples for bioanalysis, different homogenization ratios and homogenization devices were used. For example, cornea and sclera tissues are tough and not easily homogenized. If the homogenization ratio is inappropriate, the samples may become gelatinous and difficult to handle. After optimizing the homogenization ratio, a multi-functional biological sample homogenizer with three-dimensional high-speed vibration capabilities is used for sample processing, providing uniformly textured and well-flowing tissue homogenates.
Bioanalysis: Due to various physiological mechanisms and barriers, extremely low drug concentrations is suspected in some ocular tissues, which places a high demand for the sensitivity of bioanalysis. Through the development and validation of analytical methods for various tissues, we have achieved a lower limit of quantification (LLOQ) of 0.1 ng/mL, enabling effective analysis of tissue samples.
The results show that the drug concentration in plasma was extremely low (Cmax at 60.2 ng/mL). The drug mainly distributed in the vitreous , which is consistent with the pharmacokinetic characteristics of conventional intravitreal injection formulations. The study provides data support for the use of intravitreal injection as a route of administration for this drug.
Learn More
-
FAQs
-
What are ophthalmic drugs?
Ophthalmic drugs are medications that are used to treat various ocular diseases and visual impairments, including, refractive errors, cataracts, age-related macular degeneration (AMD), glaucoma, diabetic retinopathy (DR), corneal opacity, trachoma, dry eye syndrome, and conjunctivitis, etc. These drugs can be in the forms of eye drops, gels, or other medications specifically designed for ophthalmic use.
-
What are some common DMPK challenges of ophthalmic drug delivery?
The biggest challenge in ocular drug delivery research is overcoming static and dynamic biological barriers to effectively deliver the drug to target ocular tissues. These barriers can be classified based on their anatomical location and functional characteristics, which are generally categorized as anterior segment barriers and posterior segment barriers. Anterior segment barriers include static barriers, such as the cornea, conjunctiva, blood-aqueous barrier, and efflux pumps, as well as dynamic barriers, including tear drainage, and the aqueous humor. Posterior segment barriers include static barriers, such as the sclera, Bruch membrane, blood-retinal barrier, and dynamic barriers, such as the choroidal blood, etc.
-
What are the classifications of ophthalmic drugs?
1. Local-acting drugs
Primarily absorbed through the cornea/conjunctiva with none or limited systemic exposure
Metabolism occurs via ocular enzymes (e.g., esterase)
Designed primarily for ocular surface diseases (e.g., dry eye, keratitis, conjunctivitis)
2. Aqueous humor- and vitreous-targeted drugs
Distributed to intraocular tissues (e.g., ciliary body, choroid, and retina) via the dynamic pathways of aqueous humor and vitreous
Clearance depends on the circulation of aqueous humor or vitreous and the drug clearance in various distribution tissues
This is mainly designed for the treatment of glaucoma, cataract, macular diseases, and retinal diseases
-
How to deliver ophthalmic drugs to the eyes?
1. Non-invasive drug delivery methods
Eye drops: most common; rapid absorption via cornea/conjunctiva
Ointments/gels: prolonged contact time; more suitable for lipophilic drugs
2. Invasive drug delivery methods
Local ocular injections (intravitreal injection, intracameral injection, subretinal injection, subconjunctival injection, periocular injection, retrobulbar injection, etc.): direct delivery to the target tissue
Implants: Sustained-release devices
3. Systemic delivery
Oral/IV administration: Due to the blood–ocular barrier, drug concentrations in ocular tissues are limited; therefore higher doses are often required
4. Advanced methods
Contact lenses: drug-eluting lenses for controlled release
Iontophoresis: electric field-assisted penetration
Microneedles: minimally invasive suprachoroidal delivery
Related Resources




-


Approved Ophthalmic Protein Drugs and Their Preclinical Pharmacokinetic Considerations
ArticlesOct 16, 2025Learn More -


Exploring Drug Binding to Melanin: Impacts and Methods
BlogsDec 29, 2023Learn More -


What is Melanin Binding and How to Study It In Vitro?
ArticlesDec 19, 2023Learn More -


Advancing Ophthalmic Drug Development: Challenges and Strategies
BlogsNov 30, 2023Learn More -


Ophthalmic Drug DMPK Services
BrochuresNov 30, 2023Learn More -


Study on the Permeability of Ophthalmic Tissues In Vitro
PostersNov 22, 2023Learn More -


Exploration and Application of Preclinical In Vivo PK Studies for Ophthalmic Drugs
BlogsNov 17, 2023Learn More -


Advancing Ophthalmic Drug Development: Overcoming Bioanalytical Challenges in Ophthalmology
BlogsNov 17, 2023Learn More -


Ophthalmic Drugs: Preclinical Pharmacokinetic (PK) Profiles and Research Strategies
ArticlesOct 30, 2023Learn More -


Exploration and Application of Methods in Ocular In Vivo PK Studies
ArticlesOct 27, 2023Learn More -


In Vivo Bioanalysis of Ophthalmology: Challenges and Strategies
ArticlesOct 11, 2023Learn More -


Overcoming Challenges when Developing Oligonucleotides for Ophthalmic Drugs
ArticlesAug 31, 2023Learn More
Stay Connected
Keep up with the latest news and insights.