-
Overview
-
Assays
-
Regulatory Guidance
-
Experience
-
Instruments
-
FAQs
-
Related Resources
-
Related Services
Overview
The physicochemical properties of drug molecules not only restrict the interaction between drugs and their effect targets, but also affect the body's disposition and pharmacokinetic behavior of drug molecules, which is one of the key factors of the "trajectory" of drug molecules in vivo. WuXi AppTec DMPK provides high-throughput, multi-method testing services for physicochemical properties of drugs, including solubility, lipophilicity, EPSA (exposed polar surface area), pKa, solution stability, and so on.
Learn More


Assays
-
Solubility
Learn MoreType
Kinetic solubility & Thermodynamic solubility
Method
Shake-Plate method
Characteristics
High throughput
Automatic operation
-
Lipophilicity
Learn MoreType
LogD & LogP
Method
Shake-Flask method
RP-HPLC method
Potentiometric titration method
Characteristics
High throughput
Automatic operation
-
Solution Stability
Learn MoreType
Buffer stability & gastrointestinal stability
Method
Shake-Plate method
Characteristics
High throughput
Automatic operation
-
pKa
Learn MoreInstrument
Sirus T3
Method
Potentiometric titration method
UV spectrophotometry titration method
Characteristics
Automatic operation
-
EPSA
Learn MoreInstrument
UPCC-PDA/UPCC-MS/MS
Method
SFC method
Characteristics
Efficient
Accuracy
Reliability
Regulatory Guidance
The physicochemical property of drugs is one of the basis of the pharmacokinetic behavior of drugs in vivo. In general, the factors affecting the absorption process include the physicochemical properties of the drug and/or formulation factors, physiological factors, etc. The physicochemical properties of the drug include solubility, oil-water partition coefficient, pH, particle size, crystal form, permeability, and stability of the drug in the gastrointestinal tract, etc. The formulation factors include dosage form, excipients and preparation process, and the administration route of different dosage forms of the drug.
Learn More

Experience
-
150,000+
Samples
Instruments
-
-


Hamilton
-


Sirius T3
-
FAQs
-
What are the physicochemical properties of drugs?
The physical and chemical properties of drugs refer to the physical and chemical properties of drugs, including molecular size, solubility, lipophilicity, dissociation degree, polarity, crystallization state, etc. The chemical properties mainly include oxidation, reduction, and decomposition of chemical reaction characteristics. The chemical form of drugs and the interaction of the physical environment and physiological environment directly affect their chemical stability. The physicochemical properties of drugs are closely related to biochemical properties, pharmacokinetic properties, and toxicological properties, which are important aspects of drug evaluation.
-
What factors affect the physicochemical properties of compounds?
The chemical structure of a drug determines its physicochemical properties. The main factors affecting the physical and chemical properties of drugs are temperature, pH, type and content of solvents, and complex physiological environment.
-
What is the significance of the physicochemical properties of drugs for biological research?
Research on physicochemical properties of drugs in the early stage of drug development is helpful for high-throughput screening of hit compounds, guiding the optimization of lead compounds to obtain compounds with better activity and pharmacokinetic properties, and reducing the failure rate of new drug development. Early acquisition of physicochemical property parameters, on the one hand, is conducive to the optimization of biological determination methods, to ensure the accuracy of biological test data. On the other hand, it is beneficial to predict the problems encountered in vivo and in vitro processes, provide the basis for the analysis and interpretation of abnormal data in vivo and in vitro, and can guide the design of drug dosage forms and the selection of drug administration routes in the drug development stage. Therefore, the physicochemical properties of drugs are very important for biological research.
-
What are the effects of the physicochemical properties of drugs on pharmacokinetics?
The behavioral characteristics of drugs in vivo are closely related to the chemical structure and physical properties of drugs. The physicochemical properties of drugs are important factors affecting gastrointestinal absorption, such as the degree of dissociation, lipophilicity, dissolution rate, and gastrointestinal stability have different effects on gastrointestinal absorption. For insoluble drugs, the dissolution process is the limited factor of absorption. Drug distribution is a process of transmembrane transport. Most drugs pass through cell membranes through micropores or lipid bilayers in a passive diffusion way. This transport mode is closely related to the physicochemical properties of drugs, such as molecular size, chemical structure, and configuration, pKa, lipophilicity, and polarity (PSA). The physicochemical properties of drugs are also the decisive factors of drug metabolism, which determine the plasma protein binding rate, liver tissue affinity, metabolic pathway, and enzyme catalytic mechanism of drugs. At the same time, the physicochemical properties of drugs are also important factors affecting the excretion and elimination of drugs, such as the size of molecular weight and polarity directly affect the excretion pathway of drugs, fat solubility, and dissociation affect the reabsorption rate of drugs in the renal tubules. Therefore, the various physical and chemical properties of drugs are closely related and jointly affect the pharmacokinetic behavior of drugs.
Related Resources




-


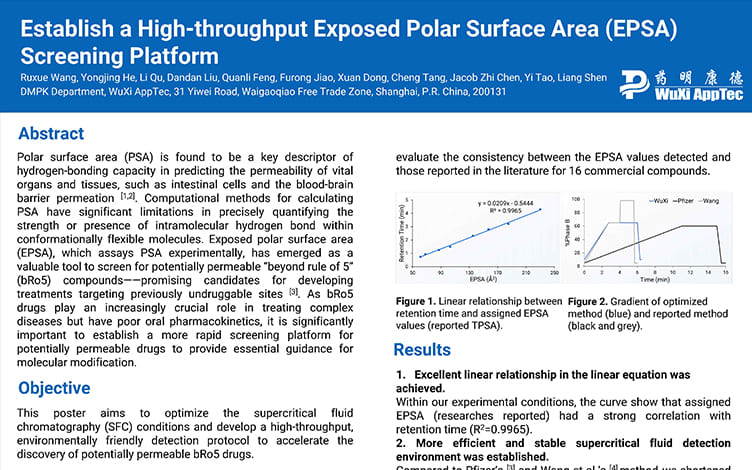
Establish a High-throughput Exposed Polar Surface Area (EPSA) Screening Platform
PostersSep 04, 2025Learn More -


Development of a High-throughput and Cost-effective Method for Experimental Polar Surface Area Measurement with Ultra-Performance Convergence Chromatography Tandem Mass Spectrometry
PostersJul 24, 2025Learn More -


Enhancing Permeability Through Exposed Polar Surface Area (EPSA) for Beyond Rule of Five (bRo5) Drug Candidates
ArticlesApr 10, 2025Learn More -


Rapid Determination of Lipophilicity: Exploration and Establishment of Reversed-Phase Liquid Chromatography (RPLC) Methods
PostersNov 05, 2024Learn More -


Ensuring drug product integrity: The crucial role of stability testing
BlogsOct 27, 2024Learn More -


How to Evaluate Lipophilicity Rapidly? The Significance of Reversed-Phase Liquid Chromatography (RP-HPLC)
BlogsDec 19, 2023Learn More -


Rapid Determination of Lipophilicity: Establishment and Application of Reversed-Phase Liquid Chromatography (RP-HPLC)
ArticlesNov 30, 2023Learn More -


Focusing on PROTAC Permeability and Solubility Improving the Oral Availability
BlogsJul 07, 2023Learn More -


Research on PROTAC Druggability: Solubility and Permeability
ArticlesJun 30, 2023Learn More
References
- 1.
Di L, Kerns E H. Drug-like properties: concepts, structure design and methods from ADME to toxicity optimization[M]. Academic press, 2015.
- 2.
《系统药代动力学》杨凌,科学出版社
Stay Connected
Keep up with the latest news and insights.