-
Overview
-
Assays
-
Case Studies
-
Experience
-
FAQs
-
Related Resources
-
Related Services
Overview
The binding of drugs to plasma proteins affects the absorption, distribution, metabolism, and excretion of drugs in the body, thereby affecting the intensity and duration of pharmacological effects. When drugs enter the human body, generally only free drugs can reach the target and exert pharmacological effects. Therefore, it is very important to determine the protein binding of drugs in drug discovery and development as well as in clinical studies. WuXi AppTec DMPK can provide customers with several methodologies for plasma protein binding, including equilibrium dialysis (HTD and RED), ultracentrifugation, ultrafiltration, flux dialysis, competitive dialysis, reporter enzyme method (for determining dynamic binding), and other methods for protein binding experiments of various matrices such as plasma, tissue, hepatocytes, and liver microsomes, and can also provide blood-plasma partition and hemolysis experiments. WuXi AppTec DMPK can test various modalities, such as plasma unstable or highly bound compounds, covalent inhibitors, peptides, Proteolysis-Targeting Chimeras (PROTACs*), siRNAs, ASOs, etc. We provide critical support for drug development by optimizing the experimental protocol to ensure accurate and reliable protein binding data.
Learn More

Assays
Case Studies
-
-

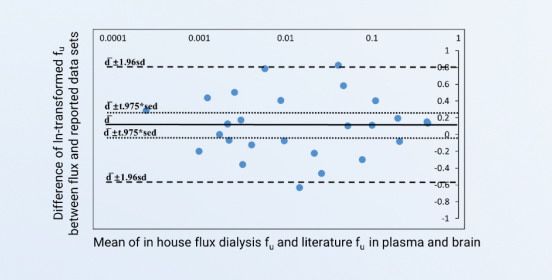
The Bland-Altman plot of fu by flux dialysis and literature-reported data
Figure 1
The dilution method is usually used for PPB determination of highly bound compounds during discovery. Since plasma dilution may lead to saturation of plasma proteins, it is recommended to use the other methods with undiluted plasma to verify the fu values in the late stages. Flux dialysis is one of the recommended methods that determine fu from the initial flux rate obtained from dialyzing commercially available compound-spiked plasma against blank plasma in in-house laboratory. It offers the advantage of measuring extremely low fu values without the effect of issues such as nonspecific binding. The figure describes a Bland-Altman plot of fu values by in-house flux dialysis and the data reported by the literature.
Figure 1. The Bland-Altman plot of fu by flux dialysis and literature-reported data, which is used to determine whether the two test methods agree. The upper and lower dashed lines are the upper and lower limit of the 95% confidence interval for the difference. The upper and lower dotted lines are the upper and lower limit of the 95% confidence interval for the mean difference. The test data is considered reliable if the mean difference 95% confidence interval encompasses zero.
-
-
For PPB determination of peptides, since they usually have large molecular weights, do not easily penetrate the dialysis membrane, and are prone to non-specific adsorption, it is recommended to use the ultracentrifugation method during drug discovery. Ten marketed peptide drugs were selected as test compounds in our laboratory. Ultracentrifugation was used to evaluate the protein binding in human plasma. The results were close to the literature values (Table 1).
Table 1. Unbound values of peptides in human plasma determined by ultracentrifugation
Sample Name
%Unbound (in-house data)
%Unbound (literature data)
Cetrorelix
14.0
14.0
Cyclo-sporine
6.8
6.8
Daptomycin
6.7
8.3
Degarelix
17.4
10.0
Eptifibatide
66.0
75.0
Icatibant
46.5
56.0
Nafarelin
33.3
20.0
Octreotide Acetate
51.1
35.0
Liraglutide
0.40
0.51
Lixisenatide
36.9
45.0
Experience
-
10+
Years
-
40K+
Compounds* species screened per year
-
≤ 5
TAT ≤ 5 Days
FAQs
Related Resources




-


What is Alpha-1 Acid Glycoprotein (AAG) and Its Role in Drug Binding for Pharmacokinetics and Pharmacodynamics Studies
ArticlesJan 09, 2026Learn More -


Evaluating Cytochrome P450 (CYP) Enzyme Inhibition: Hepatocytes vs. Liver Microsomes
BlogsOct 31, 2025Learn More -


Fast Biomimetic High-Performance Liquid Chromatography Method for Evaluating Human Plasma Protein Binding
PostersSep 30, 2025Learn More -


Biomimetic Chromatography Method to Estimate Human Plasma Protein Binding for Peptides
PostersSep 19, 2025Learn More -


Decoding the Key Role of Plasma Protein Binding in Drug-Drug Interactions
ArticlesAug 15, 2025Learn More -


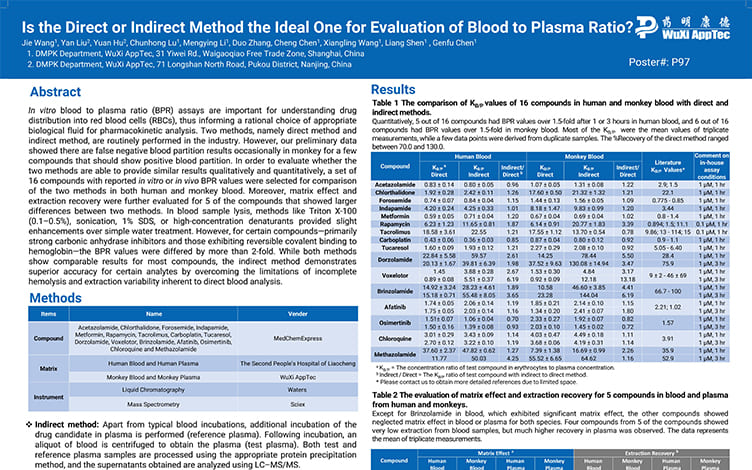
Is the Direct or Indirect Method the Ideal One for Evaluation of Blood to Plasma Ratio?
PostersAug 06, 2025Learn More -


The Determinants in Evaluating siRNA Plasma Protein Binding Using Agarose Gel Electrophoretic Mobility Shift Assay (EMSA)
PostersDec 03, 2024Learn More -


Does the Flux Competition Method Add Significant Value in Species Difference Protein Binding Evaluation?
PostersNov 21, 2024Learn More -


Effects of Lipoprotein Binding and Plasma Dilution on the Accuracy of Ultracentrifugation Method in Plasma Protein Binding
PostersNov 13, 2024Learn More -


Plasma Protein Binding of Oligonucleotide Drugs: Implications, Methods and Strategies
ArticlesJun 27, 2024Learn More -


Ultra-high Throughput Mass Spectrometry Based Analysis by Direct Injection (Echo® MS) for Plasma Protein Binding Assay
PostersMay 24, 2024Learn More -


Rapid EMSA: A New Method of Testing PPB in Oligonucleotides
BlogsApr 30, 2024Learn More -


Experimental Validation of the Reliability of Dilution Method for Plasma Protein Binding Assay in Human Plasma Using Commercial Compounds
PostersApr 18, 2024Learn More -


Plasma Protein Binding of Oligonucleotides Using Rapid Agarose Gel Electrophoretic Mobility Shift Assay
PostersMar 15, 2024Learn More -


Why Use Flux Dialysis Methods to Measure Plasma Protein Binding (PPB) of High Protein-binding Drugs
BlogsJan 31, 2024Learn More -


Exploring Drug Binding to Melanin: Impacts and Methods
BlogsDec 29, 2023Learn More -


Plasma Protein Binding (PPB) Measurement of High Protein-binding Drugs: Principles and Advantages of the Flux Dialysis Methods
ArticlesDec 29, 2023Learn More -


Plasma Protein Binding of Nine Antisense Oligonucleotides by Ultrafiltration
PostersDec 19, 2023Learn More -


What is Melanin Binding and How to Study It In Vitro?
ArticlesDec 19, 2023Learn More -


Establishment of the Flux Dialysis Method for Highly Bound Compounds in Human Plasma and Rat Brain Homogenate
PostersDec 15, 2023Learn More -


The Role and Challenges of Plasma Protein Binding in Oligonucleotide Drug Development
ArticlesNov 22, 2023Learn More
Stay Connected
Keep up with the latest news and insights.