-
Fast and Reliable Data Delivery
-
High-Throughput Automation Platforms
-
Experienced and Professional Teams
-
High Standard Instruments and Facilities
-
Integrated Service
Fast and Reliable Data Delivery
-
Fast Data Delivery
No lead time for in vitro and in vivo assays
Less than five working days for screening assays
TAT 4 months for regular IND-enabling package
-
Reliable Experimental Results
Meet IND and NDA requirements of FDA, NMPA and EMA
Successfully pass over 15 years of audits
Digital operation and management platform
Precise and intelligent operation system, efficient and safe data delivery
Project Management System
Compound Management System
Electronic Lab Notebook System
Report Automation System
QuickTracer Tracking System
-
Precise and intelligent process management
Systematic management from contract signing to project delivery makes the process more transparent and more intelligent.
Automated and efficient experiment management
Automatically creates millions of sample labels every year and scans codes to capture the dynamic trajectory of samples.
Safe and compliant authority management
The system has a clear layered structure with authority limitations, so all users operate only within the scope of their authorization.

-
Compound Storage Services
With a storage throughput of over one million compounds and a historical management record of more than one million compounds, the management platform integrates automated storage equipment with compound management systems to provide clients with safe, accurate, and efficient compound management services.
100% Recording Accuracy of Compounds
The compounds are weighed on a high-precision balance with real-time transmission and recording of weighing data. Tens of thousands of weighing times occur per month, but scientists can accurately retrieve the inventory and usage of compounds.
100% Accuracy of Aliquot and Solution Preparation
The Hamilton automatic workstation calculates the required amount of solvent according to the instructions and automatically prepares the solution with a target concentration and aliquot. Real-time data synchronization guarantees that the process is more reliable.

-
Real-time Recording of Operating Traces
An audit trail system can record all online operation traces from users in real-time, ensuring accurate and safe records.
Standard Recording Template
The customized recording system was developed to precisely match DMPK experimental types and achieve online recording. The sample code can be acquired with one click instead of manual editing, having accurately generated sample codes for over 150,000 cumulative projects.
Secure Data Backup
VERITAS NetBackup software enables incremental backup of electronic data daily and full weekly backup, so data is secured and recoverable.

-
Comprehensive Coverage of Assay Types
Various automated programs aid in the efficient writing of reports, thereby accelerating the assay process.
Reduction of Human Writing Errors
To reduce human errors, copying and pasting was eliminated from copy and paste steps which occurred in traditional editing.
Man-machine Collaboration to Ensure Quality
The human-computer synergy mode not only improves writing efficiency by 50%, but also allows scientists to shift their focus of work from data alignment to data plausibility, which doubles the accuracy of data and reports.

-
Tracking System
Display project progress in total transparency and real-time with one click on your PC or mobile phone.

View More


High-Throughput Automation Platforms

To deliver high-throughput and fast testing services, WuXi AppTec DMPK continues to leverage the potential of automation. We have successfully validated and deployed several high-throughput automation platforms, including in vitro ADME high-throughput integrated automated workstation, biological sample pre-treatment full-automation system, smart compound library, automated blood sampler and plasma separation systems, intelligent animal facility and scheduling system, etc. to reduce the human error, improve data quality, and shorten the turnaround time, thus accelerating clients' development process.
Experienced and Professional Teams
18+
Years of experience
1,000+
Employees
1,600+
Global clients
1,700+
Successful IND filings supported
200+
Assay types
300,000+
In vitro experiments conducted per year
20,000+
In vivo experiments conducted per year
180,000+
Samples analyzed per week
View More About Our Teams


-
Comprehensive Services with Streamlined Communications
We have a specialized and dedicated service model. Each client will be connected to a dedicated study director who will provide comprehensive management services for the pharmacokinetic project from drug discovery to the clinical phase. Our Study Director, with a professional background in pharmacokinetic research and extensive drug development experience, collaborates with senior DMPK experts and functional heads to provide comprehensive project management and technical support. This ensures the high-quality and efficient project delivery.

-
Innovation and Development
Our team of scientists continues to strive to improve our research capabilities and broaden our scope of drug-enabling platforms through innovative ideation and partnership. We have established pharmacokinetic research strategies and test service systems for each type of featured drug modality(such as therapeutic proteins, peptides, oligonucleotides, antibody-drug conjugates (ADCs), proteolysis-targeting chimeras (PROTACs*), etc.).
The findings and results were reported in scientific articles in peer-reviewed journals, international academic conferences, and patent applications.

Posters
-


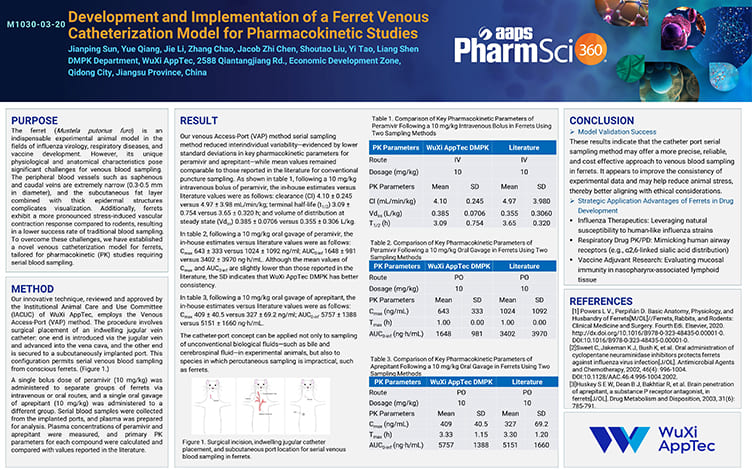
Development and Implementation of a Ferret Venous Catheterization Model for Pharmacokinetic Studies
Nov 28,2025
-


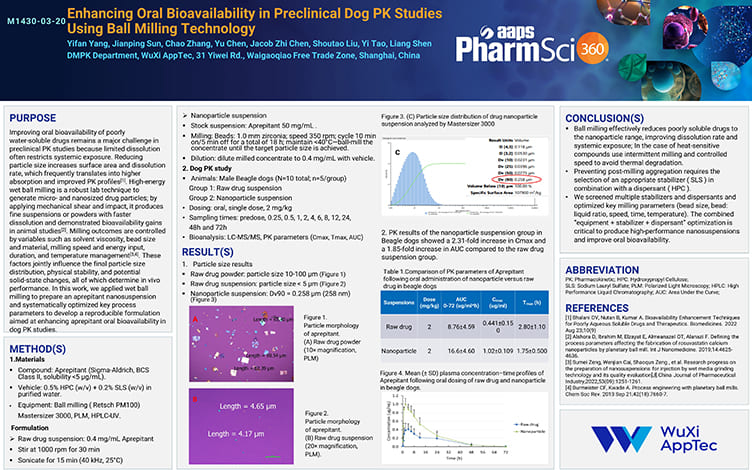
Enhancing Oral Bioavailability in Preclinical Dog PK Studies Using Ball Milling Technology
Nov 28,2025
-


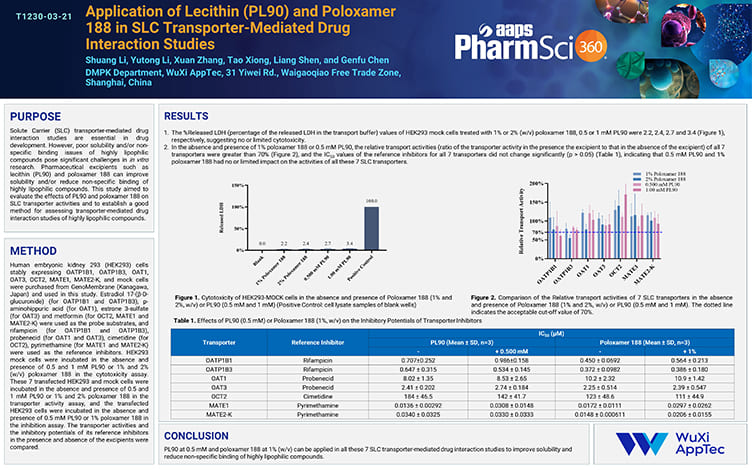
Application of Lecithin (PL90) and Poloxamer 188 in SLC Transporter-Mediated Drug Interaction Studies
Nov 21,2025
View Less


High Standard Instruments and Facilities
We provide comprehensive pharmacokinetic services from early screening, and preclinical development, to clinical research through high-standard instruments and facilities including industry-leading Liquid Chromatography-Mass Spectrometry (LC-MS), Ligand Binding Assay (LBA) platforms, high-resolution mass spectrometry (HRMS), data processing software, radioactivity detectors, etc. Our animal facilities are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC International).
-
Non-GLP BA
-
MetID
-
Radiolabeled ADME
-
High Standards of Animal Facilities
-
Non-GLP BA
Leveraging liquid chromatography-mass spectrometry (LC-MS), ligand binding assay (LBA), and automated instruments, we offer high-throughput and efficient qualitative and quantitative bioanalysis services. This supports the research of candidate drugs, metabolites, biomarkers, and large molecules in in vivo and in vitro samples from screening to the IND filing stage.
-
Mass Spectrometer
-
Ligand Binding
-
Nucleic Acid Detection qPCR
-
Full automation, High-throughput
-
-


Waters Vion™ QTof
-


Orbitrap Eclipse™ Tribrid™
-


Sciex Triple Quad™ 7500+
-


PE NexION 2000
-
-
-


MESO Sector S 600MM
-


BD LSRFortessa™ X-20
-


Molecular Devices SpectraMax M5e
-
-
-


QIAcube HT
-


QuantStudio™ 7 Flex Real-Time PCR System
-


QIAgility
-
-
-


Apricot Designs Dual Arm
-


High-throughput intelligent delivery system
-
-
-
MetID
The team is equipped with industry-leading high-resolution mass spectrometry platforms, such as Thermo Scientific™ Orbitrap Eclipse™ Tribrid™ and Waters®VionTM IMS QTof. In addition, professional data processing softwares are available within the team, including Thermo Scientific™ Compound Discoverer™/Thermo Scientific™ BioPharma Finder™, UNIFI®, and Mass-MetaSite (Molecular Discovery).
-
High-Resolution Mass Spectrometry
-
Equipment for Radioactivity Detection
-
-


Orbitrap Eclipse™ Tribrid™
-


Orbitrap Exploris™ 480
-


Q-Exactive™ HF
-


VION™ IMS QTof
-


Q-Exactive™ Plus
-


Q-Exactive™
-


LTQ Orbitrap XL
-


Xevo®G2 QTof
-
-
-


Solid scintillation counter: off-line detection of radioactivity
-


Liquid scintillation counter: total radioactivity detection
-


On-line detection of radioactivity
-
-
-
Radiolabeled ADME
Utilizing radiolabeled compound synthesis techniques and radioactive detection equipment, we offer pharmacokinetic studies of test drugs marked with radioactive isotopes.
-
Radiolabeled ADME
-
-


Q-Exactive™ HF
-


Orbitrap Exploris™ 480
-


Thermo LTQ-Orbitrap
-


SCIEX API 6500
-


Liquid Scintillation Counter
-


β-RAM Online Radioactivity Detectorβ-counter
-


Solid Scintillation Counter
-


Cryostat Microtome
-


Multifunctional Laser Imager
-


Liquid Scintillation Counter
-


Triple Quad MS
-


1H NMR
-
-
-
High Standards of Animal Facilities
The Animal facilities of WuXi AppTec DMPK are in Shanghai, Suzhou, Nantong, and Nanjing, respectively. All facilities are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC International). Animal barrier facilities in Shanghai, Suzhou, and Nantong have obtained the OLAW-approved Animal Welfare Assurance.
-
Animal Facilities
-
Integrated Service
Comprehensive Support to Accelerate New Drug R&D Process
WuXi AppTec DMPK works closely with other departments at WuXi AppTec to offer a one-stop service to clients.
WuXi AppTec is a trusted partner and contributor to the pharmaceutical and life sciences industries, providing R&D and manufacturing services that help advance healthcare innovation. With operations across Asia, Europe, and North America, we offer integrated, end-to-end services through our unique CRDMO (Contract Research, Development, and Manufacturing Organization) platform. We are privileged to work alongside nearly 6,000 partners across 30+ countries, supporting their efforts to bring breakthrough treatments to patients.

Our Vision
Every drug can be made and every disease can be treated.
Integrated, End to end enabling Platform
~6,000
Innovative customers enabled
30+
Customers from more than 30 countries
-
Chemistry Platform (WuXi Chemistry)
-
Biology Discovery Platform (WuXi Biology)
-
Testing Services Platform (WuXi Testing)
View More


Stay Connected
Keep up with the latest news and insights.