-
Overview
-
Study Models and Platforms
-
Experience
-
Study Strategies and Assays
-
Instruments
-
Case Study
-
FAQs
-
Related Resources
-
Related Services
Overview
Benefited from our extensive experience with oligonucleotide studies, combined with guidelines issued by various drug regulatory authorities, industry white papers and studies in frontier literatures, WuXi AppTec DMPK has established a set of pharmacokinetic evaluation system tailored for oligonucleotide drugs1,2. We can offer a comprehensive list of in vitro assays and in vivo PK experiment designs, and can conduct mass balance and tissue distribution studies with radioisotope labeling, provide diversified quantitative analysis methods for oligonucleotides, and possess the ability to identify metabolites. Our advantages in setting up preclinical PK strategy, data interpretation and cross-departmental cooperation, can effectively shorten the development cycle of oligonucleotide drugs.
Learn More

Study Models and Platforms
-
Comprehensive capabilities with an integrated bioanalytical platform
Pharmacokinetic/pharmacodynamic studies of oligonucleotides require a diverse array of bioanalytical platforms to support the measurement of plasma/tissue concentration, tissue distribution, concentration of oligonucleotide loaded into the RNA-induced silencing complexes (RISC), knockdown efficiency of targeted mRNAs and proteins. We have extensive experience and comprehensive instruments to provide a wide range of bioanalysis solutions for different types of oligonucleotides, from early-stage drug screening to IND-filing.

-
In vitro ADME study platform
Oligonucleotides can be metabolized by ubiquitous nucleases through the body. In vitro experiments to investigate the stability of oligonucleotides in blood, target tissues, and lysosomes/tritosomes can support the evaluation of their stability in the circulatory system and targeted organs, and help optimize their chemical modifications. Additionally, plasma protein binding results can assist in understanding their PK/PD relationship. WuXi AppTec DMPK has successfully established a variety of in vitro stability models for oligonucleotides, including plasma, serum, liver and kidney S9 and homogenates, hepatocytes, lysosomes and other matrices, also established PPB assay using different methods (ultrafiltration, ultracentrifugation and electrophoretic mobility shift sssay).

-
In vivo PK studies of oligonucleotides
Currently the most common systematic administration methods for oligonucleotides are intravenous and subcutaneous injection, while local administration methods include intrathecal administration, and intravitreal injection. Compared to conventional small molecules, the concentration of oligonucleotides in the target tissue (such as liver) has a much better correlation with the efficacy of oligonucleotides than the plasma concentration. WuXi AppTec DMPK can conduct in vivo PK, tissue distribution, excretion studies of oligonucleotides with different routes of administration in mice, rats, and monkeys. In particular, we had established a mature method of intrathecal injection of oligonucleotides with high a success rate, as well as liver biopsy techniques to support the PK studies of target tissues in monkeys.

-
Oligonucleotide metabolite profiling and identification platform
Well in vitro-in vivo correlation of metabolism experimental system, including different in vitro metabolic incubation systems, and in vivo plasma, targeted tissue and excreta metabolism studies. With flexible optimization of analytical conditions, our team of experienced scientists use both manual screening techniques and oligonucleotide-specific software (Thermo Scientific’s BioPharma Finder) to perform metabolite profiling and identification. We have the capability to run 100 + MetID studies each year for oligonucleotide, including a variety of ASOs and siRNAs with different chemical modifications and delivery systems.

Innovative Models | Predict siRNA Metabolism
A novel study published in the DMD journal identifies optimal in vitro metabolic models, such as heparinized plasma and pH-adjusted liver systems, to predict GalNAc-siRNA metabolism in vivo, enhancing the accuracy of preclinical metabolism study for therapies like Inclisiran.
Experience
-
5+
Years’ experience in oligonucleotide drug development
-
20+
IND filing molecules
-
70+
Global clients of oligonucleotides
Study Strategies and Assays
-
There are various types of oligonucleotides, and their pharmacokinetic properties vary depending on mechanism, delivery system, and chemical modification. In the preclinical development stage, study strategies should be customized according to the specific characteristics of the oligonucleotide to accelerate its development. For in vitro ADME study, it is necessary to select an appropriate in vitro metabolic model based on the structural characteristics of the drug; for in vivo PK study, different administration methods can be used to investigate the PK properties. An appropriate detection method should be developed in accordance with the properties of the oligonucleotide.

EXPLORATORY Lead Molecule Optimization | PRE-CLINICAL Support Clinical Candidate Molecule Characterization and IND Applications | CLINICAL Support Clinical Development and NDA Applications | |
ADME | Plasma protein binding S9 metabolic stability Plasma/serum stability Tissue homogenate metabolic stability Lysosome/tritosome metabolic stability Hepatocyte stability Plasma/tissue homogenate/S9/hepatocyte metabolite identification and profiling | Plasma protein binding Tissue homogenate metabolic stability Plasma/serum stability S9 metabolic stability and metabolite identification ADME study with radiolabeled compounds in toxicological species Identification of metabolites from in vivo samples (tissue, plasma, urine, feces, bile, cerebrospinal fluid, etc.) | Identification of metabolites in human plasma, urine, and feces, etc. Identification of metabolites from in vivo samples such as tissue, plasma, urine, feces, and bile in toxicological species |
DDI | Inhibition of drug transporters CYP enzyme inhibition (reversible and time-dependent inhibition) CYP450 enzyme induction | Prediction of DDI risks in human through PBPK modelling Clinical DDI study | |
PK | Tissue distribution in rodents Biomarker detection in PK or PD species | PK study and tissue distribution in rodents Study on urine, feces, and bile excretion in rodents PK study and tissue distribution in non-rodents | In vivo PK study Immunogenicity evaluation In vivo population PK study |
Instruments
-
-


Sciex Triple QuadTM 6500+
-


Q-ExvtiveTM Plus
-


Fluorescence Detectors
-


Molecular Devices SpectraMax M5e
-


QuantstudioTM 7 fLEX
-
Case Study
-
Integrated bioanalysis of GalNAc-conjugated siRNAs
Background: Cemdisiran is an siRNA conjugated with N-acetylgalactosamine (GalNAc) with an asymmetrical RNA/dTdT overhang at the 3′ end of the AS. It targets the C5 complement pathway and treats complement-mediated diseases by inhibiting the hepatic production of C5 complement proteins.
In vivo studies: We carried out a PK study in mice according to the conventional early in vivo PK protocol of siRNAs. Plasma, serum, and liver samples were collected and analyzed at multiple time points after a single administration by subcutaneous injection. The specific study design is shown in Figure 1: Three dose groups of low, medium, and high were set, and the mice were tranquilized to collect biological samples at 1 and 6 h and on days 1, 2, 3, 7, 14, and 21 after a single dose.
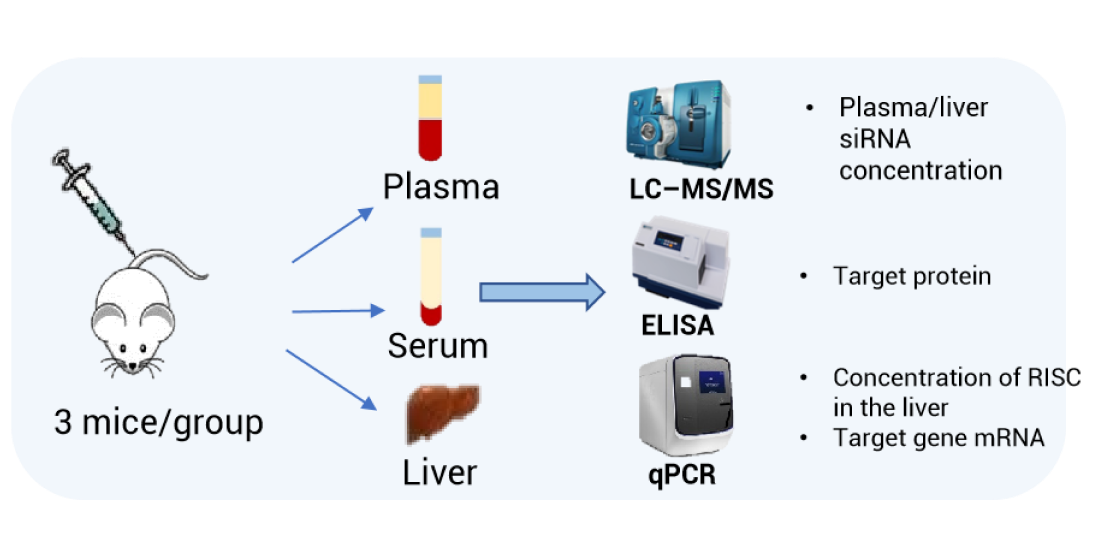
Figure 1. Cemdisiran mouse PK study design and bioanalytical solutions
In this study, three different bioanalytical platforms and four bioanalytical methods were used to analyze the samples:
(1) The plasma and liver concentrations of the siRNA were quantified using LC-MS/MS (Figure 2).
(2) Levels of C5 complement protein in serum were detected using ELISA (Figure 3).
(3) The RISC concentration in the liver was determined using RNA immunoprecipitation combined with stem-loop RT-qPCR.
(4) Target gene expression levels were determined in the liver using stem-loop RT-qPCR.
Results: By detecting the RISC concentration in liver tissue and comparing it with the inhibition rate of the target protein or the degradation rate of the target mRNA (Figure 4 and 5), it can be seen that the RISC concentration, rather than the drug concentration in free plasma, is more closely related to the gene silencing effect in target. We successfully applied suitable bioanalysis platforms to support the PK/PD studies of oligonucleotides.
Learn More
-

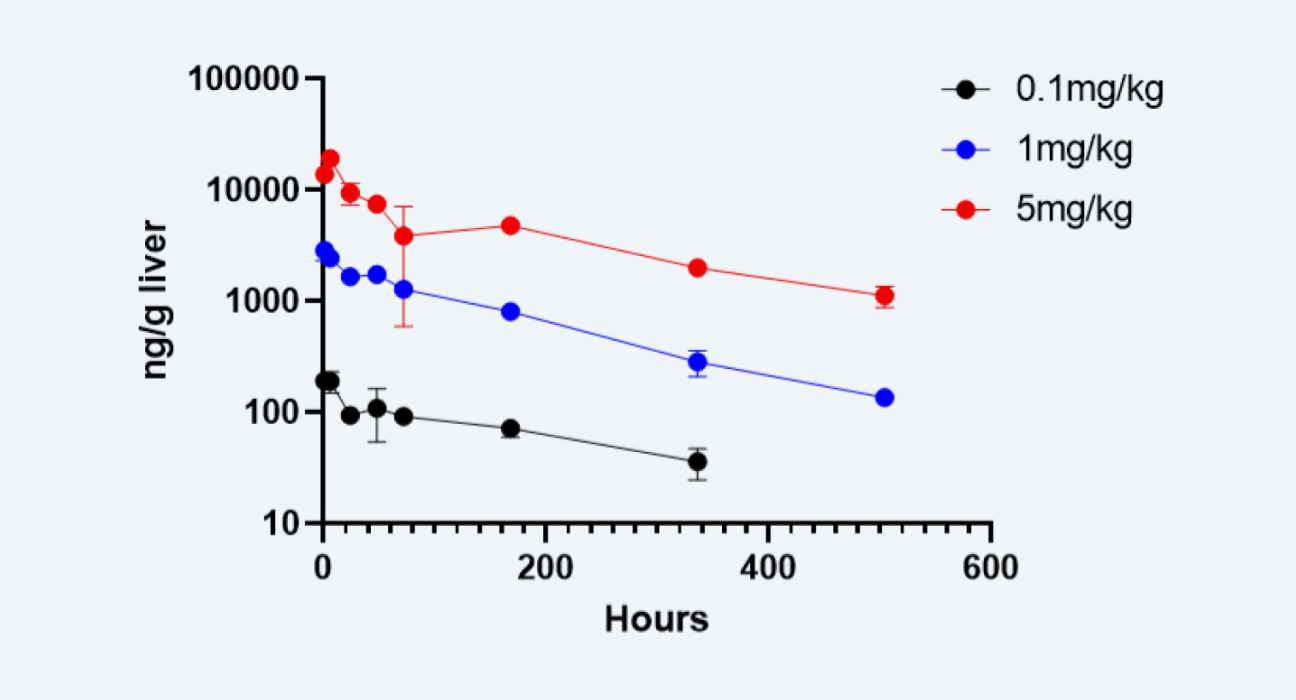
Determination of the Cemdisiran concentration in the livers of mice in different dosage groups by LC-MS/MS
Figure 2
-

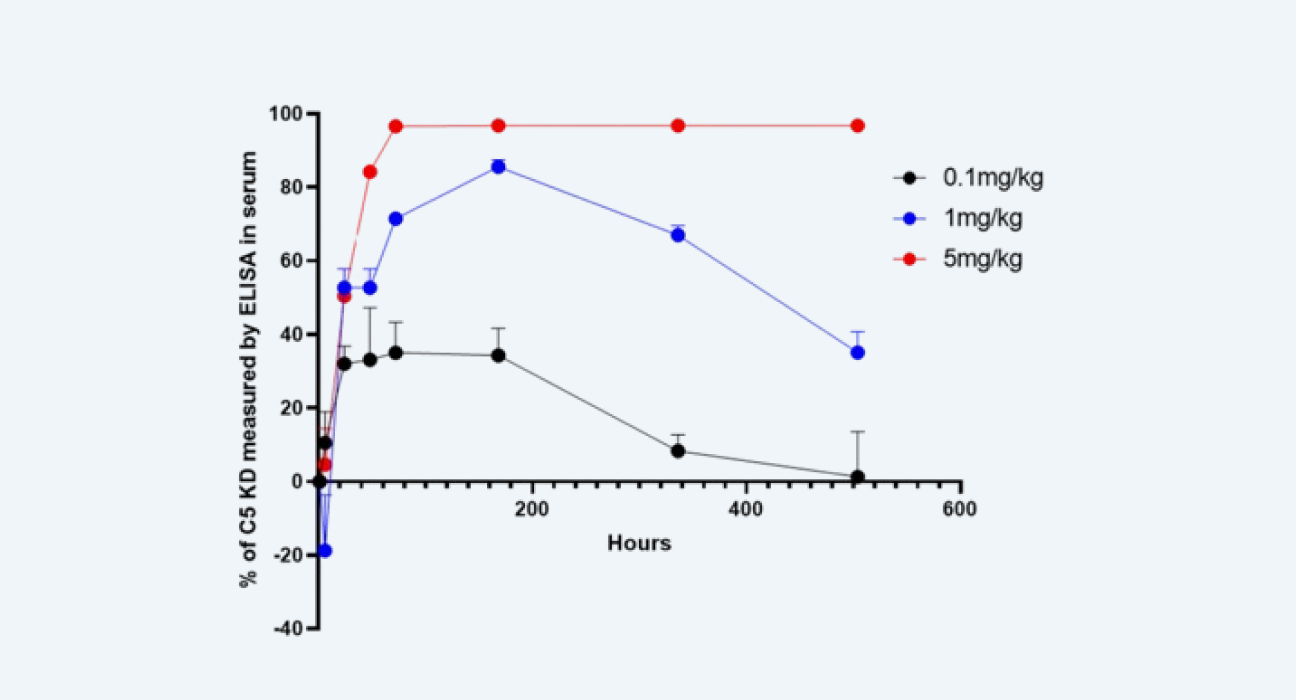
Determination of C5 complement protein levels in serum using enzyme-linked immunosorbent assay (ELISA)
Figure 3
-

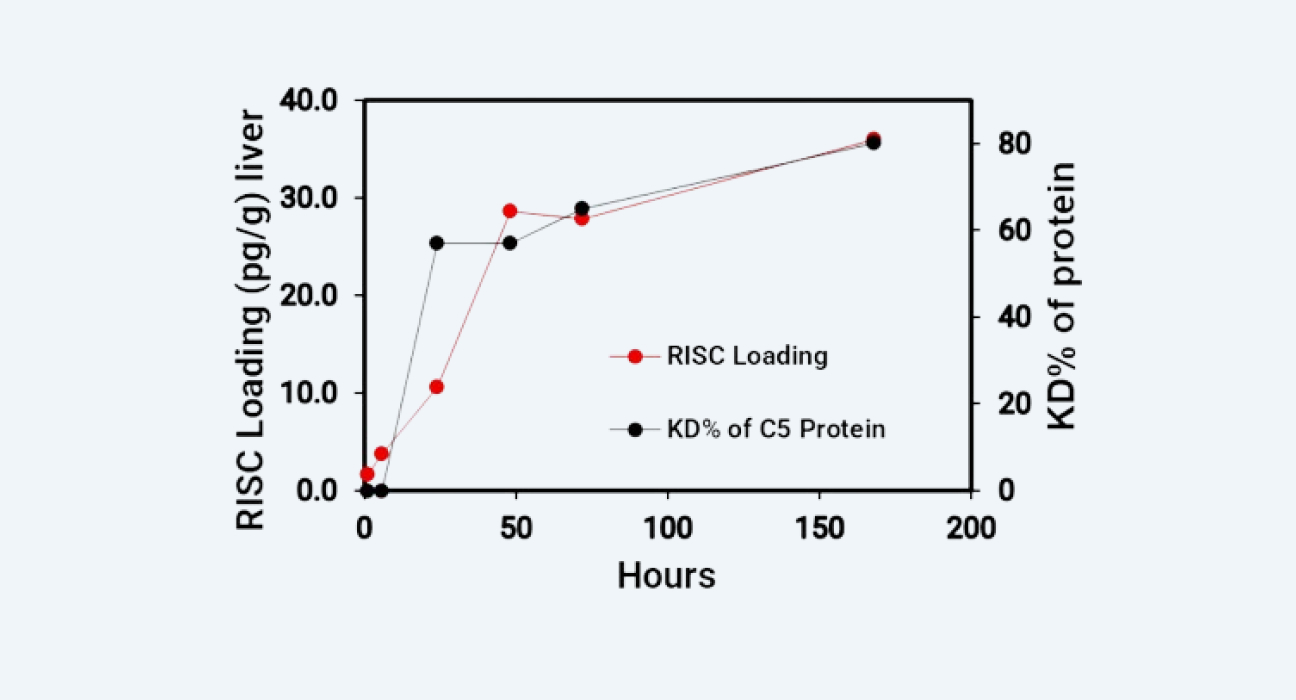
RISC concentration in mouse liver vs. C5 complement protein inhibition rate
Figure 4
-

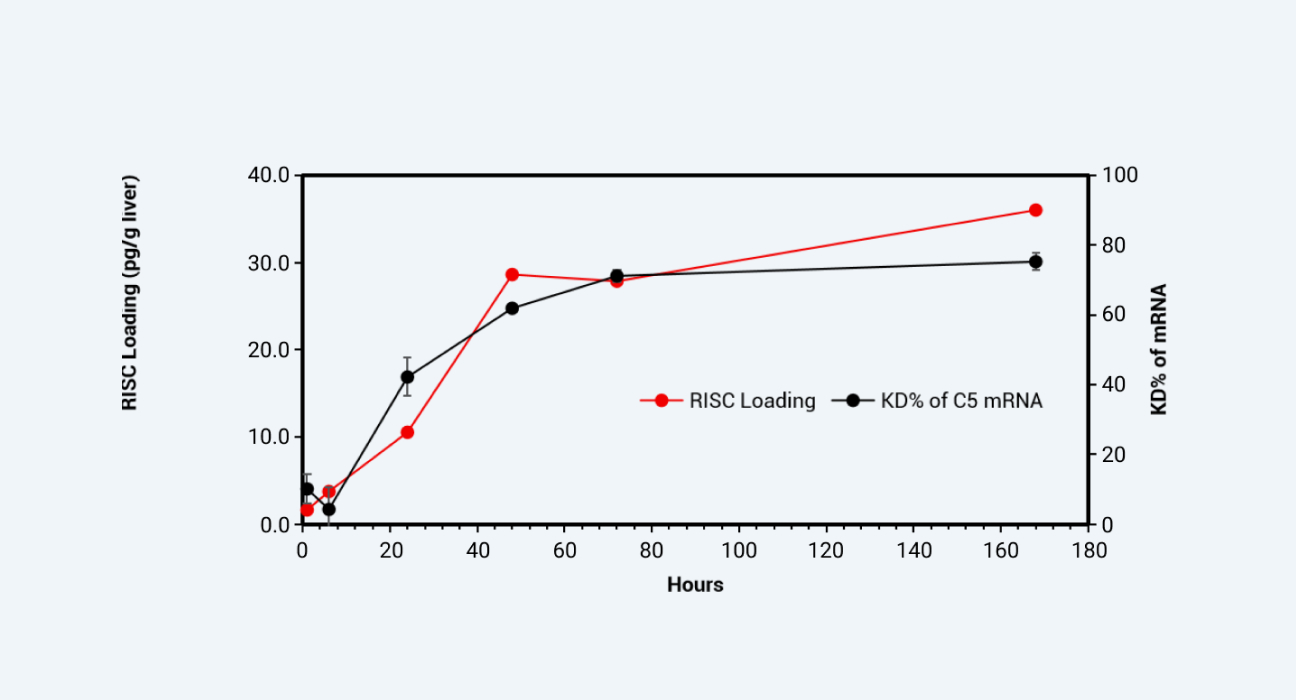
RISC concentration in mouse liver vs. target gene mRNA degradation efficiency
Figure 5
-
FAQs
-
What are oligonucleotide drugs?
Oligonucleotide drugs are therapeutic agents based on synthetically modified nucleic acids that can modulate gene expression via a range of processes, which primarily include small interfering RNA (siRNA), antisense oligonucleotide (ASO), aptamers, and miRNA mimics or inhibitors. They have the potential to target traditionally undruggable disease-causing genes and patient-specific sequences relevant to rare diseases.
-
What studies are recommended for the early DMPK evaluation of oligo molecules?
The early DMPK evaluation of Oligo can be roughly divided into in vitro stability studies of different models and in vivo tissue distribution +PKPD studies in pharmacodynamic related species. It’s recommended to use platform-based approach for the oligo drug development. For well-established platform (such as GalNAc-siRNA) that already has more mature modification and delivery system, some studies can be postponed or only need to be characterized at the IND filing stage. For oligo with new chemical modifications or delivery platforms, in vitro stability experiments, metabolite identification, and PKPD experiments will have more significance to shed light on the lead optimization in the early stage.
-
How to select the animal species in the preclinical PK studies of oligo?
Considering the gene homology reason and consistency of oligo’s metabolism across species, we can use either mouse or rat in rodent studies depending which one is more pharmacodynamically related, but most frequently select monkey in the large animal studies because their PK is more predictive of humans.
-
What is the difference between an oligonucleotide and a nucleotide?
The main difference between nucleotides and oligonucleotides lies in their structure and applications. A nucleotide is the basic unit of nucleic acids (DNA/RNA), composed of a phosphate group, a sugar molecule, and a nitrogenous base. Oligonucleotides are short synthetic polymers of nucleotides (typically 13-25 nucleotides in length) that are used in research and medicine, including applications like PCR amplification, diagnostic assays, and targeted therapies. While nucleotides serve as fundamental building blocks in biological systems, oligonucleotides are designed tools for specific scientific and medical purposes.
Related Resources




-


Deciphering Nucleoside and Analogs: Mechanisms, Landscape, and Their Phosphates Analysis based on LC-MS/MS
ArticlesFeb 27, 2026Learn More -


What Are Antibody–Oligonucleotide Conjugates (AOCs) and Their Structural Characteristics
ArticlesNov 28, 2025Learn More -


Sensitive LC-MS/MS Method for Cationic Ligand-Conjugated siRNA Therapeutics
PostersNov 08, 2025Learn More -


Overcoming Challenges in Oligonucleotide Metabolism with Innovative Solutions -WuXi AppTec DMPK TechTalk
VideosNov 07, 2025Learn More -


Optimisation of an In Vitro Plated Monkey Hepatocyte Model and Comparative Metabolite Profiling of a GalNAc-Conjugated siRNA, siRNA01, in Monkey Liver Homogenate and Hepatocytes
PostersSep 26, 2025Learn More -


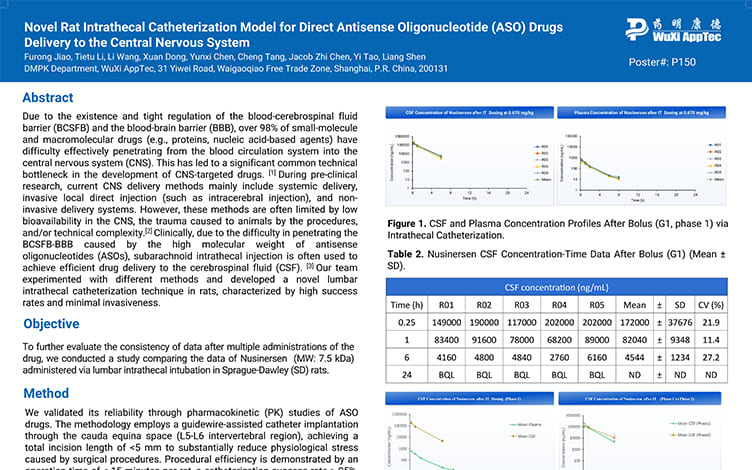
Novel Rat Intrathecal Catheterization Model for Direct Antisense Oligonucleotide (ASO) Drugs Delivery to the Central Nervous System
PostersAug 28, 2025Learn More -


Advancing Novel Therapeutics with Innovative DMPK Strategies in Metabolism and Bioanalysis
WebinarsAug 22, 2025Learn More -


Factsheet-Intrathecal Administration in Rats and Monkeys
BrochuresAug 08, 2025Learn More -


An Efficient and Environmental Friendly Method for the Determination of Nucleoside Phosphate Compounds by Using LC-MS/MS
PostersAug 01, 2025Learn More -


Optimization of Sample Pretreatment Methods for the Determination of an Oligonucleotide by Using LC-MS/MS
PostersJul 17, 2025Learn More -


In Vitro Metabolism Methods for Oligonucleotides
White PapersJun 20, 2025Learn More -


How to Analyze and Separate Oligonucleotides and Active Metabolites Using LC-MS/MS
ArticlesJun 20, 2025Learn More -


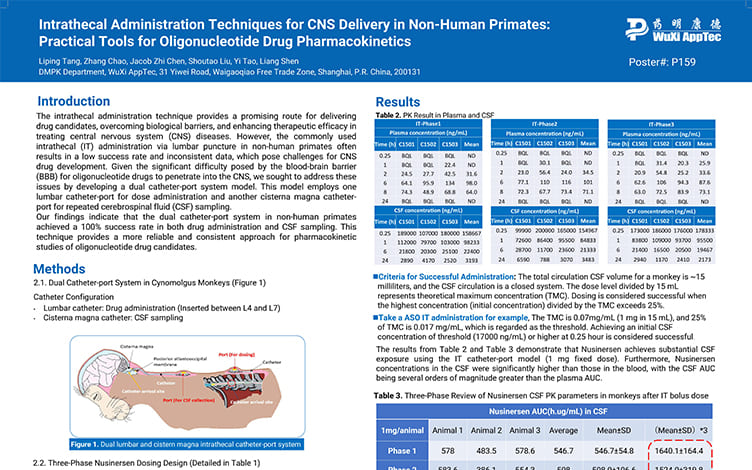
Intrathecal Administration Techniques for CNS Delivery in Non-Human Primates:Practical Tools for Oligonucleotide Drug Pharmacokinetics
PostersJun 05, 2025Learn More -


Advancing In Vitro Metabolic Models and Metabolite Identification for Oligonucleotide Therapeutics
WebinarsJun 05, 2025Learn More -


Unlocking Reliable In Vitro Metabolic Models for Oligonucleotide Therapeutics with the Latest Publication on DMD
BlogsMay 28, 2025Learn More -


Intrathecal Administration: Breaking Barriers for Central Nervous System Drugs
VideosMay 28, 2025Learn More -


Comparative Metabolism of a GalNAc-Conjugated siRNA, Inclisiran, Among Various In Vitro Systems and Correlations with In Vivo Metabolism in Rats
PublicationsMay 08, 2025Learn More -


FAQs on Oligonucleotide DMPK Studies Stability, Distribution, Administration, and Bioanalysis
BlogsApr 03, 2025Learn More -


Navigating Complexities in Oligonucleotides: DMPK Strategies and CNS-Targeting Solutions
WebinarsFeb 20, 2025Learn More -


Methods and Strategies for In Vitro Metabolism Studies of Oligonucleotides
ArticlesJan 08, 2025Learn More -


Comparative Metabolite Profiling and Identification of a GalNAc-Conjugated siRNA, siRNA01, in Plasma Prepared with Various Anticoagulants, Serum, and In Vivo Plasma Using LC-UV-HRMS
PostersOct 25, 2024Learn More -


Validation and Comprehensive Bioanalytical Strategy of In Vivo Studies for RNAI Therapeutics in Early Discovery
PostersSep 13, 2024Learn More -


8 In Vitro Systems for Oligo Metabolism Study: Pros and Cons and Selection Suggestions
ArticlesAug 25, 2024Learn More -


Intrathecal Antisense Oligonucleotides: PK and Strategy
ArticlesJul 04, 2024Learn More -


Quantitative Analysis of a GalNAc-siRNA Conjugate and Its Metabolites Using Two-Dimensional Liquid Chromatography Coupled with Tandem Mass Spectrometry
PostersJul 04, 2024Learn More -


Plasma Protein Binding of Oligonucleotide Drugs: Implications, Methods and Strategies
ArticlesJun 27, 2024Learn More -


Rapid EMSA: A New Method of Testing PPB in Oligonucleotides
BlogsApr 30, 2024Learn More -


Plasma Protein Binding of Oligonucleotides Using Rapid Agarose Gel Electrophoretic Mobility Shift Assay
PostersMar 15, 2024Learn More -


Metabolite Identification of Nucleoside Analog Prodrugs in Biological Matrix by Derivatization Coupled with Radio-Detector and Mass Spectrometry
PostersMar 07, 2024Learn More -


Quantitative Analysis of GalNAc-siRNA Conjugates in Tissue Homogenate Using Two-Dimensional Liquid Chromatography Coupled with Tandem Mass Spectrometry
PostersFeb 22, 2024Learn More -


Development of a Plasma Stability Assay of Oligonucleotides
PostersFeb 12, 2024Learn More -


A Comprehensive Bioanalytical Strategy of In Vivo Studies for RNAi Therapeutics in Early Discovery
PostersJan 31, 2024Learn More -


Effect of pH on Metabolite Profiling and Identification of GalNAc Conjugated siRNA in In Vitro Metabolic System
PostersJan 23, 2024Learn More -


Plasma Protein Binding of Nine Antisense Oligonucleotides by Ultrafiltration
PostersDec 19, 2023Learn More -


The Advantage of LC-MS Method for Specific siRNA and It's Shortmer Quantitation to Support Mouse Liver Pharmacokinetics Study
PostersNov 30, 2023Learn More -


Unveiling the Future of Oligonucleotide Drugs: Overcoming Bioanalytical Challenges
BlogsNov 22, 2023Learn More -


The Role and Challenges of Plasma Protein Binding in Oligonucleotide Drug Development
ArticlesNov 22, 2023Learn More -


Why Use Liquid Chromatography-Mass Spectrometry (LC-MS) for Quantitative Pharmacokinetic Analysis of Oligonucleotides
BlogsNov 09, 2023Learn More -


Exploring Oligonucleotide Drugs: An Introduction and Overview of Pharmacokinetic and Bioanalytical Strategies
ArticlesNov 09, 2023Learn More -


Metabolite Profiling and Identification of Oligonucleotide in In Vitro Metabolic System
PostersOct 30, 2023Learn More -


Why Integrated Bioanalysis Strategies are Crucial for Non-clinical Early In Vivo PK Studies of siRNAs
BlogsOct 30, 2023Learn More -


Quantitative Analysis of Oligonucleotides: The Strength of RT-qPCR
BlogsOct 20, 2023Learn More -


Overcoming the Challenges of Metabolite Identification and Profiling for Developing Oligonucleotides
BlogsOct 20, 2023Learn More -


Application of Liquid Chromatography-Mass Spectrometry (LC-MS) in Oligonucleotides Quantitative Analysis
ArticlesOct 19, 2023Learn More -


How to Use Ligand Binding Assays (LBA) for DMPK Quantitative Analysis of Oligonucleotide
BlogsOct 10, 2023Learn More -


What You Need to Know About the ADME Characteristics of EMA/FDA Approved siRNA Drugs?
BlogsOct 03, 2023Learn More -


Integrated Bioanalysis Strategies in Non-clinical Early In Vivo PK Studies of siRNAs
ArticlesSep 25, 2023Learn More -


DMPK Quantitative Analysis of Oligonucleotides Using RT-qPCR
ArticlesSep 20, 2023Learn More -


Exploring the ADME Characteristics of FDA Approved ASOs: Essential Insights for You
BlogsSep 20, 2023Learn More -


Oligonucleotide Drugs: Strategies for Metabolism and Metabolite Profiling and Identification
ArticlesSep 13, 2023Learn More
References
- 1.
Berman, C. L., Antonsson, M., Batkai, S., Bosgra, S., Chopda, G. R., Driessen, W., Foy, J., Hassan, C., Hu, X. S., Jang, H. G., Meena, Sanseverino, M., Thum, T., Wang, Y., Wild, M., & Wu, J. T. (2023). OSWG Recommended Approaches to the Nonclinical Pharmacokinetic (ADME) Characterization of Therapeutic Oligonucleotides. Nucleic acid therapeutics, 33(5), 287–305. https://doi.org/10.1089/nat.2023.0011
- 2.
Guidance, D. Clinical Pharmacology Considerations for the Development of Oligonucleotide Therapeutics. Guidance for Industry. (2022)
Stay Connected
Keep up with the latest news and insights.