-
Overview
-
Assays
-
Study Strategies
-
Experience
-
FAQs
-
Related Resources
-
Related Services
Overview
For the test compounds in the early screening stage, the unsatisfactory physical and chemical properties, the insufficient compound amount, and the tight time are commonly encountered. To deal with these challenges, the WuXi AppTec DMPK established a team for preclinical formulation screening for PK studies, pharmacodynamic, and toxicological studies. The team has more than ten years of experience and can perform rapid formulation screening and preparation services for large and small animal experiments. They also provide more than nearly 1,000 arms of formulations for preclinical animal experiments every week. They can use limited (milligram) compounds to offer a suitable formulation for preclinical animal experiments within 24 hours.
Learn More


Assays
The formulation team provides formulation screening and formulation services for pharmacokinetic studies from the screening stage to the IND application stage. It provides formulation screening services for pharmacodynamic tests and preclinical toxicological experiments. The team can select suitable excipients to dissolve the compound to tackle the solubility issue according to the physical and chemical properties of each specific drug substance (such as dissociation constant pKa, lipophilicity, aqueous solubility). The most suitable formulation for a drug substance is selected by in vitro solubility assessment, homogeneity, and particle size analysis. Further screening is performed from eligible candidates to ensure the formulation with better in vivo bioavailability or good achievement of experimental objectives.
Learn More

Study Strategies
The formulation team can select vehicles that meet the requirements of the PK study within one working day. In formulation screening, solubilization technology (such as pH adjustment and cosolvent) is a commonly used technical means. For some poorly soluble compounds, a cosolvent plus surfactant or complexing agent plus pH adjustment are typically used in combination. In addition, the DMPK formulation team can customize the formulation screening strategy according to differentiated project needs
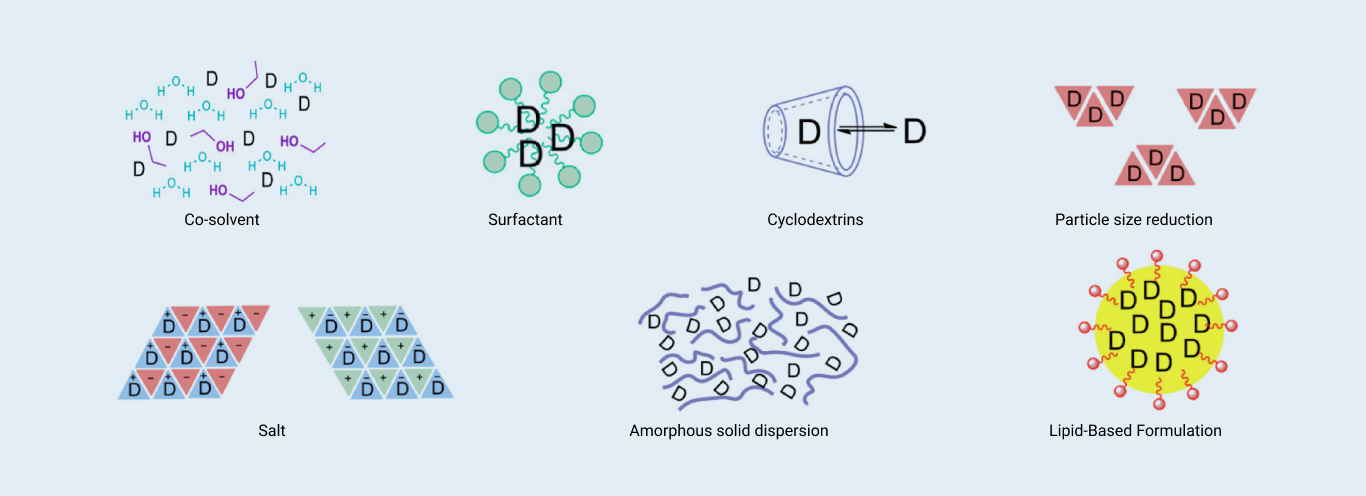
Common methods for preclinical formulation screening
Experience
-
12+
Years of experience in preclinical vehicle screening
-
1000+
Bottles per week of formulation screening and preparation
-
10000+
Vehicle safety database
FAQs
Related Resources




-


Particle Size Analysis and Reduction Techniques to Enhance Drug Bioavailability in Preclinical Studies
ArticlesSep 05, 2025Learn More -


Evaluation of the Safety and Cross-Species Tolerability of Solutol HS 15 via Intravenous Administration in Various Animals
PostersAug 28, 2025Learn More -


Minimizing Hemolysis in In Vivo Pharmacokinetic Studies: Causes, Assessment, and Optimal Vehicle and Additive Selection
ArticlesAug 08, 2025Learn More -


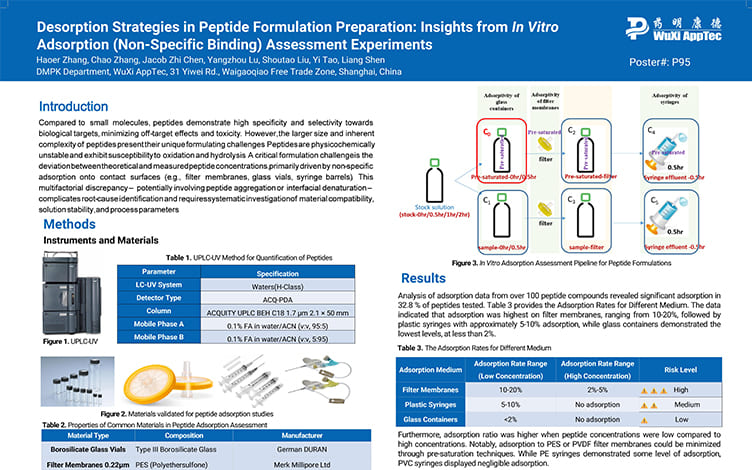
Desorption Strategies in Peptide Formulation Preparation: Insights from In Vitro Adsorption (Non-Specific Binding) Assessment Experiments
PostersJul 17, 2025Learn More -


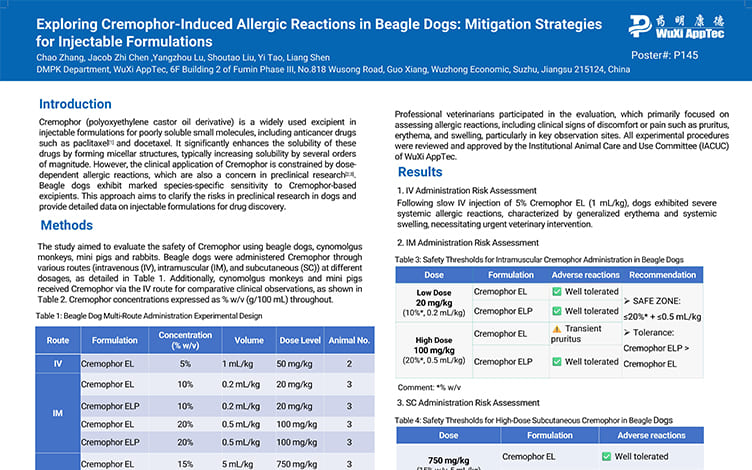
Exploring Cremophor-Induced Allergic Reactions in Beagle Dogs: Mitigation Strategies for Injectable Formulations
PostersJun 12, 2025Learn More -


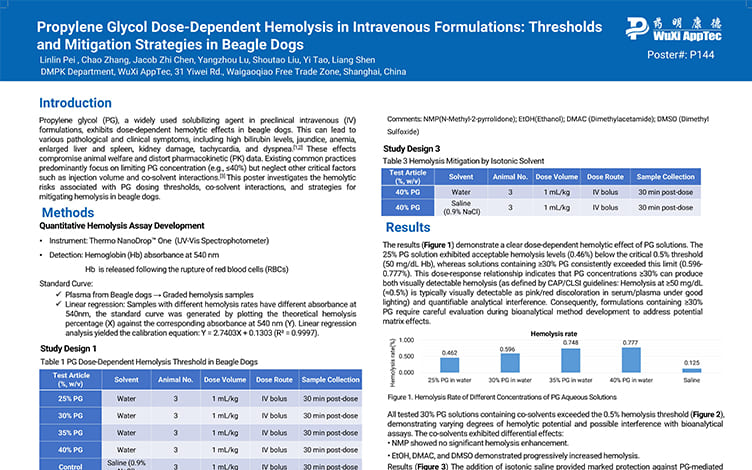
Propylene Glycol Dose-Dependent Hemolysis in Intravenous Formulations: Thresholds and Mitigation Strategies in Beagle Dogs
PostersJun 05, 2025Learn More -


The Impact of Gastrointestinal pH on Oral Drug Absorption
ArticlesMar 28, 2025Learn More -


Food Effects on Oral Drug Absorption: Preliminary Investigation of Mechanisms and Case Studies in Large Animal Pharmacokinetics
ArticlesJul 26, 2024Learn More -


Formulation Development Strategy: Preclinical PK, PD, and TK Considerations
ArticlesMay 31, 2024Learn More -


Self-Emulsifying Drug Delivery System (SEDDS): Enhancing the Oral Absorption of Lipophilic Compounds
BlogsApr 18, 2024Learn More -


How to Improve the Bioavailability of Poorly Water-Soluble Compounds?
BlogsApr 03, 2024Learn More -


Enhancing the Bioavailability of Poorly Soluble Compounds: Strategies for Formulation Optimization in Preclinical Studies
ArticlesMar 15, 2024Learn More -


Strategies for Enhancing Oral Exposure of Water-Insoluble Compounds
PostersFeb 05, 2024Learn More
Stay Connected
Keep up with the latest news and insights.