-
Overview
-
Study Models and Platforms
-
Strengths
-
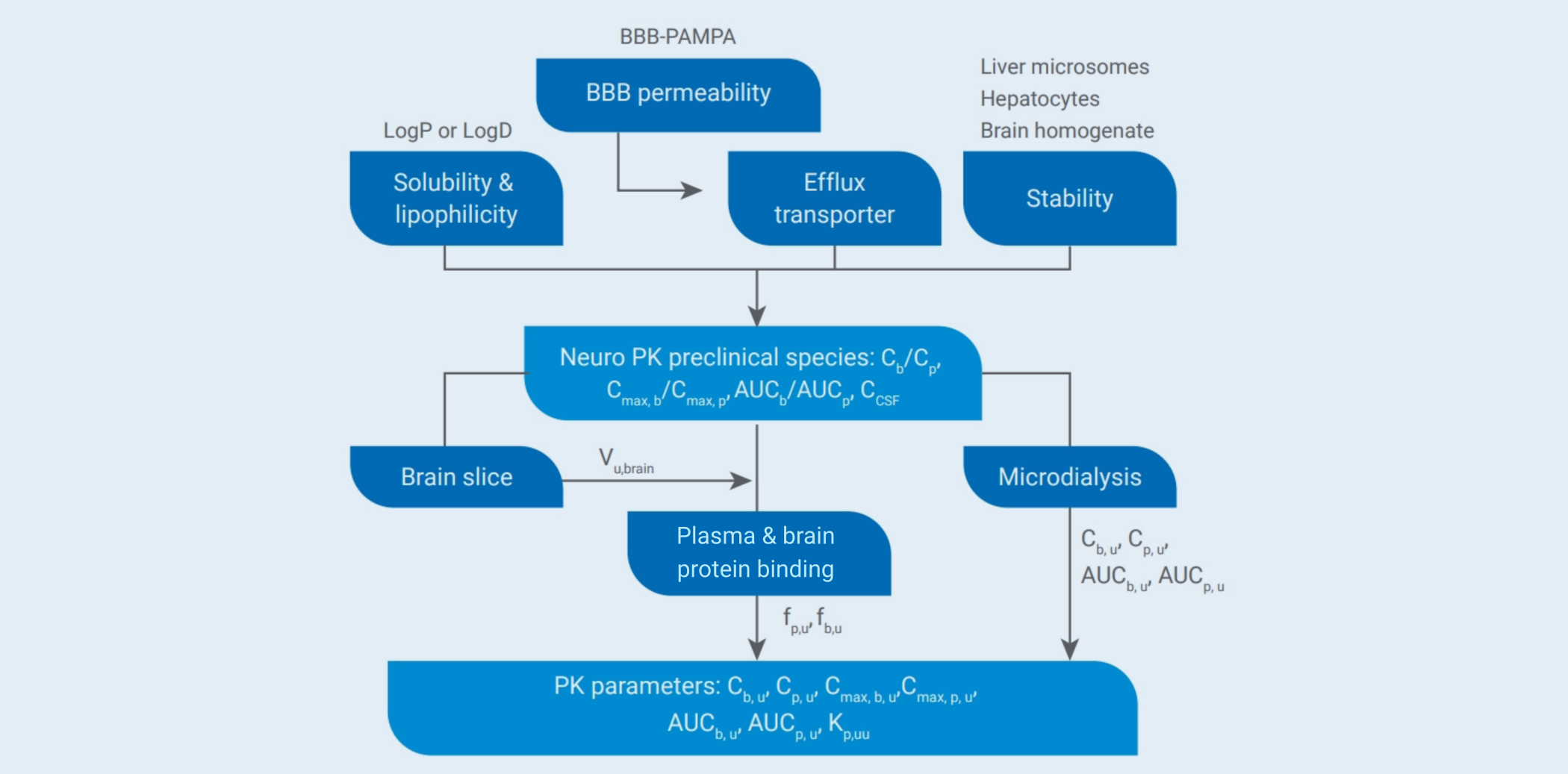
Study Strategies and Assays
-
Case Study
-
FAQs
-
Related Resources
-
Related Services
Therapeutic Areas
Overview
The success rate for CNS drug development is very low (~ 8%). It’s critical to use accurate and rapid evaluation assays to select more promising drug candidates. With years of experience, a series of CNS-specific capabilities have been established and routinely performed in DMPK, such as a distinctive and accurate “Funnel” model to evaluate brain penetration of CNS drugs in vitro, routine or brain-specific administration routes including lateral ventricle administration and intrathecal injection, serial collection of CSF from rats and large animals, precise separation and collection of ~20 kinds of brain regions, and quantitative whole body autoradiography (QWBA) to exhibit a whole picture of brain distribution. Our experiences are not only on small molecules, but also on proteins, oligonucleotides, etc. in both screening and IND filling projects.
Learn More

Study Models and Platforms
-
“Funnel” model for in vitro brain permeability evaluation of small molecules
This model accurately differentiated 10 CNS drugs that could penetrate the brain and 14 drugs with poor passive diffusion ability and/or as substrates for efflux transporters among 24 commercially available drugs.

-
Brain administration routes
In addition to conventional administration routes, direct brain delivery such as lateral ventricle administration and intrathecal injection in both rodents and large animals have been successfully established.
-
Microdialysis technology
Microdialysis in large and rodent animals helps to obtain Kp,uu directly in vivo without performing in vitro protein binding assays.

-
Serial CSF collection
The cannulation or puncture models of both rodents and large animals, including rats, monkeys, and dogs, enable serial CSF collection, which reduces animal number and individual variation.

-
Precise brain region collection
Approximately 20 brain regions can be precisely collected. Drug distribution and exposure in each brain region can be individually evaluated.
Species
Olfactory bulb
Cerebral cortex
Thalamus
Hypothalamas
Hippocampus
corpus striatum
diencephalon
Cerebellum
Pons
Medulla oblongata
substantia nigra brainstem
dorsal root ganglion
dura mater
caudate nucleus
amygdaloid body
cingulate cortex
Gray matter in the cortex
White matter in the cortex
Rat
Mouse
Monkey
Dog
Strengths
-
15+
Years of experience
-
20
Precise brain regions collection
-
6+
Diverse CNS administration and sampling techniques
-
“Funnel” model
Distinctive “Funnel” model
Study Strategies and Assays
-
Lipophilicity is a crucial parameter for brain penetration of small-molecule drugs. However, molecules with high lipophilicity are often the substrates of efflux transporters or metabolized rapidly. Therefore, accurate in vitro assessment models, appropriate parameter ranges, and screening strategies are crucial for selecting promising candidates. We have established a comprehensive CNS drug property evaluation platform. The “stepwise” screening strategy enables rapid, accurate, and efficient assessments of CNS drugs, facilitating the identification of CNS candidate drugs with good brain permeability and stability.
Case Study
-
Brain microdialysis of acetaminophen in rat
Take a commercial compound acetaminophen as an example. The combination of brain microdialysis and intravenous blood microdialysis (Figure 1) enabled the data sampling from the same animal to get free drug concentrations in the brain and plasma, unbound AUC, and Kp,uu. The data from microdialysis technology was compared with the general in vivo and in vitro combination methods.
Analyte: Acetaminophen
Animal Model: brain microdialysis + intravenous blood microdialysis + carotid artery cannula (CAC), male SD rats (n=4)
Sample collection: After intravenous (IV) administration, the dialysates in the brain and blood were continuously collected at selected time points using microdialysis technology. Serial blood samples were collected from CAC.
Bioanalysis: The concentrations of the test compound in brain and blood dialysate samples from the microdialysis method were determined by the LC-MS/MS method; The total concentrations of the test compound in serial plasma samples from CAC were determined by the LC-MS/MS method as well.
The results showed that the free drug concentrations determined via the intravenous blood microdialysis method matched well with the data coming from the serial bleeding method (after correction with plasma fraction unbound obtained from the in vitro plasma protein binding assay), see Figure 2. The free unbound brain-blood AUC ratio was calculated based on concentrations of free compound in brain and plasma (Figure 3), the validation ratio (0.774) was similar to the data reported in the literature (0.8232). The application of microdialysis technology can simultaneously monitor free drug concentrations in brain and blood from the same animal, unbound AUC and Kp, uu can also be calculated. It is of great value to evaluate the relationship between plasma PK and target concentration in CNS drug research.
Learn More
-

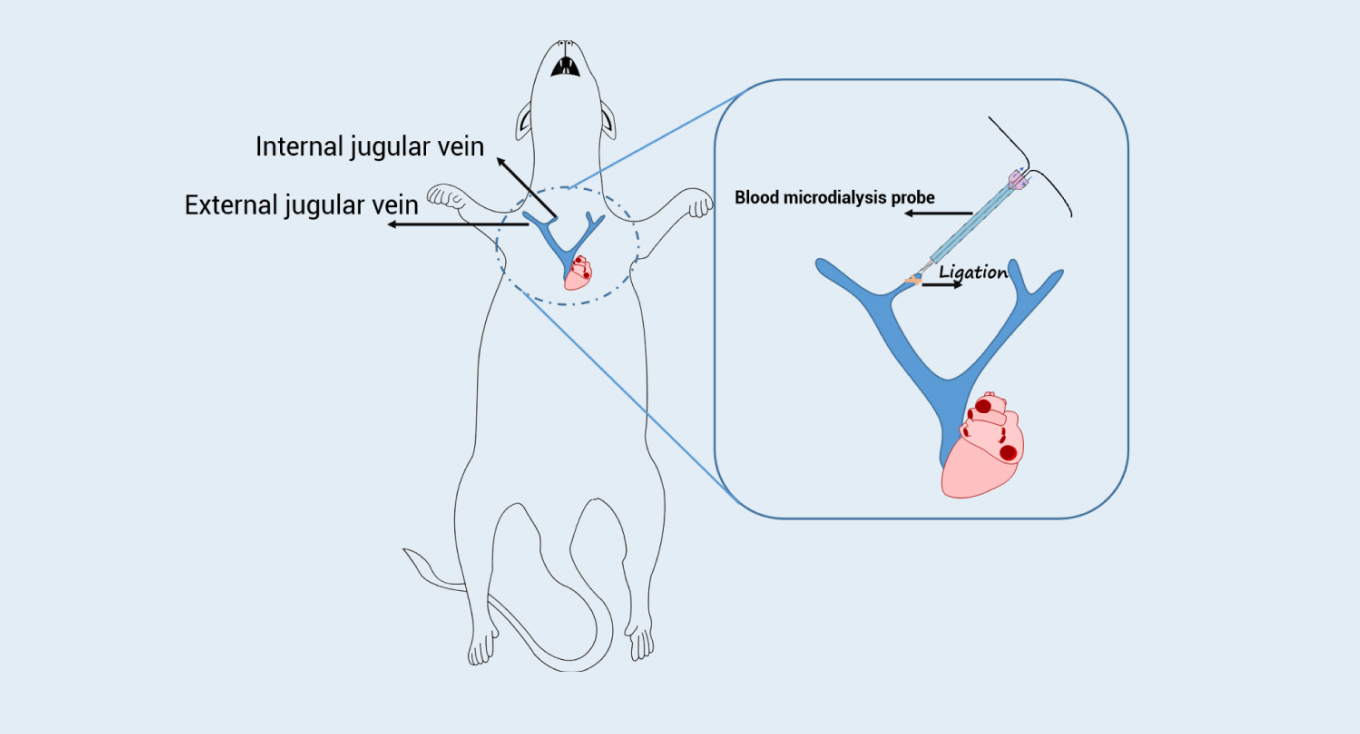
Schematic diagram of intravenous blood microdialysis
Figure 1
-

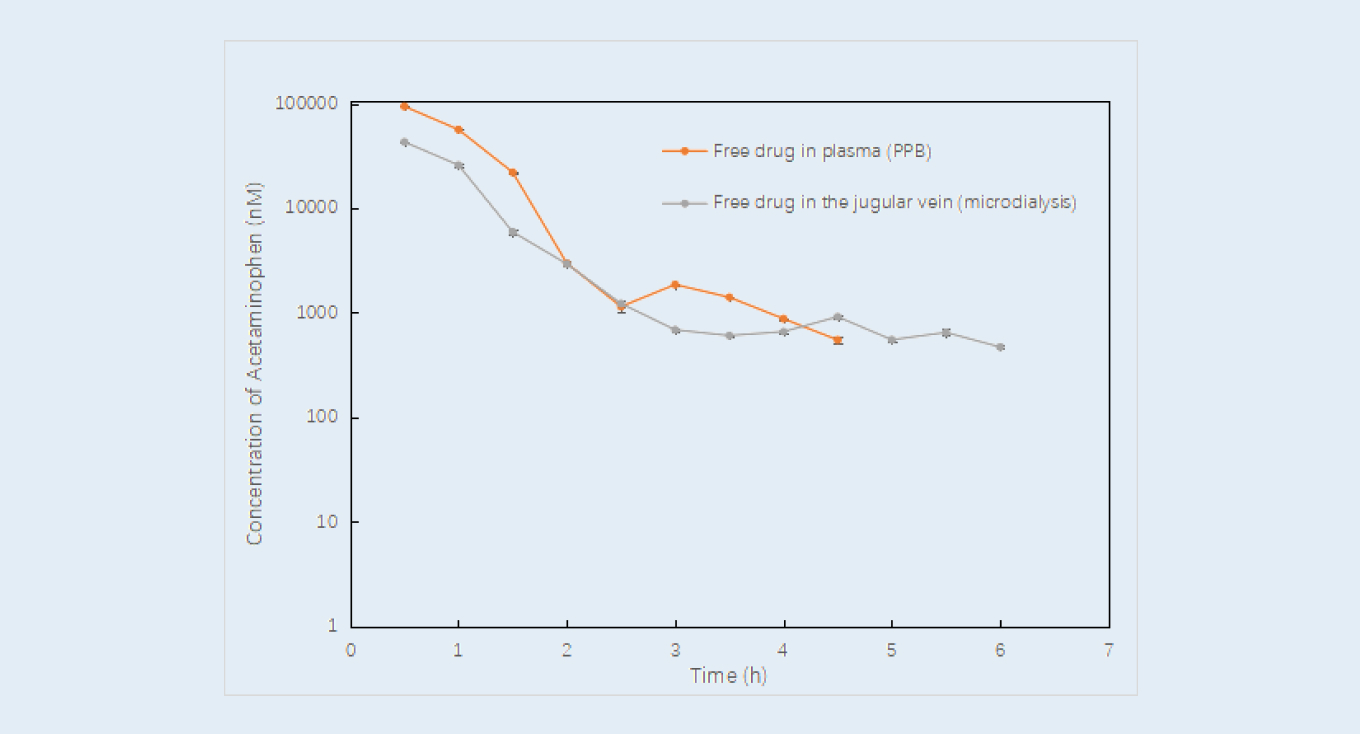
Comparison of free drug concentration in plasma obtained via the serial bleeding method and the microdialysis method
Figure 2
-

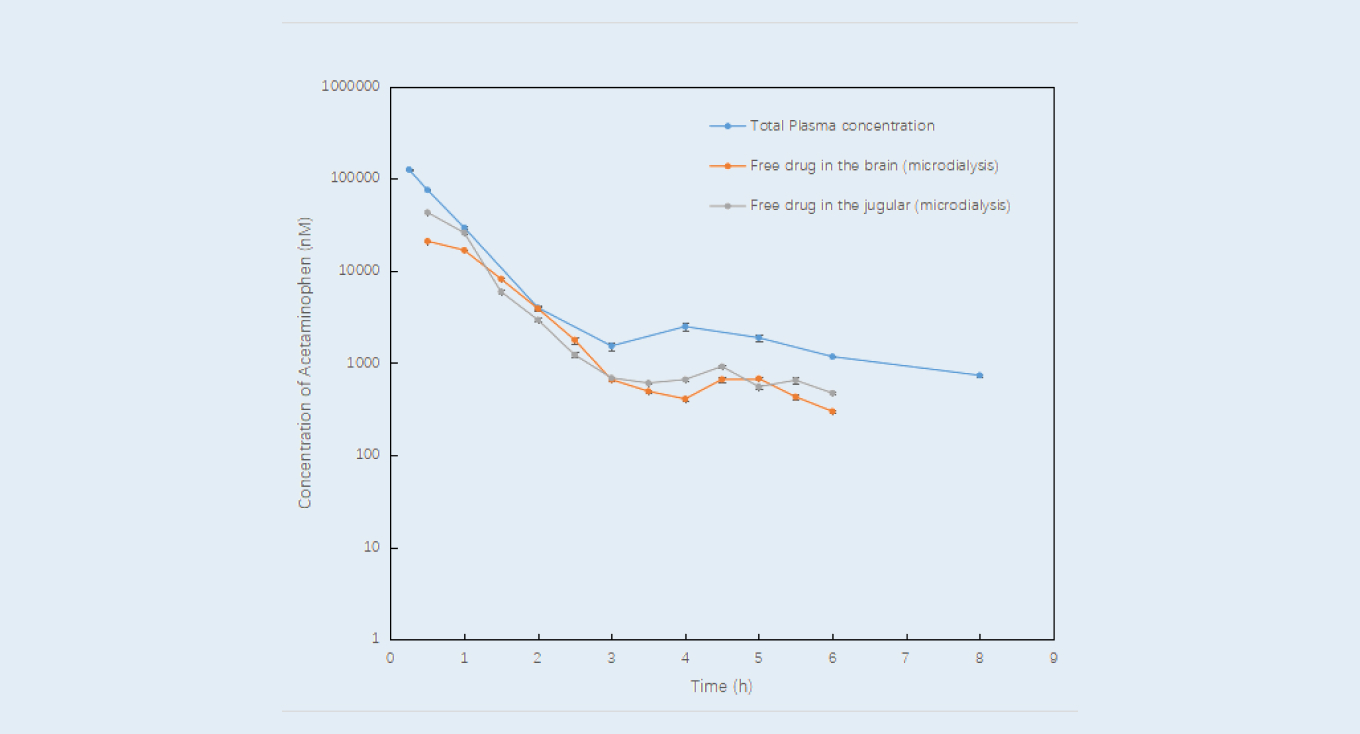
Brain microdialysis in combination with intravenous blood microdialysis
Figure 3
-
FAQs
-
If a higher total brain/plasma ratio means a better CNS penetration?
No. The total blood/plasma ratio is calculated using the total drug concentrations in brain and plasma. However, the unbound brain/plasma ratio (Kp, uu or Cb, u/Cp, u) should be used to evaluate the drug penetration to BBB. The total concentration could be largely influenced by non-specific binding of compounds to proteins and lipids in plasma or brain homogenate.
-
Can brain fraction unbound (fb,u) be used as an informative parameter during structural optimization?
No. The fb, u values are not for structural optimization of compounds, as when fb,u changed, the Cb,t (Cb,u =Cb,t * fb,u) may be changed as well when the structure changes. Therefore, in vitro plasma protein binding assay and brain tissue homogenate binding assay are not recommended to serve as one of the parameters for compound screening at early stage.
-
Can CSF concentration be used as a surrogate for CISF for all small molecules?
CCSF is considered to be within three-folds range of CISF (obtained through microdialysis detection) if a transporter is not involved. However, it’s not always accurate to use CSF concentration to represent CISF, especially for compounds that are transporters’ substrates. In addition, CSF is not a well-mixed compartment and is turned over rapidly (every 5 hours). CSF concentrations may vary significantly at different sampling sites.
-
Are large animals (e.g. monkeys) more reliable to predict human CNS penetration?
For compounds that are not substrates of efflux transporters, Kp,uu is similar in different species, and rat (or other species) Kp,uu can be used to predict free concentration in human brain with the following conversion:
[Cb,u,human ≈ Kp,uu,rat * Cp,u,human ]
For compounds that are transporters’ substrates, monkeys will be considered as a better species for human brain exposure prediction due to species similarity in transporters.
Related Resources




-


Tackling the CNS Drug Challenges with DMPK Toolkit
WebinarsDec 05, 2025Learn More -


Optimization and Assessment of Intrathecal (IT) Administration to Improve Drug Delivery to the CNS in Rats
PostersOct 31, 2025Learn More -


Establishment of a Novel Funnel Model for Evaluating Blood-Brain Barrier Penetration In Vitro
PostersSep 30, 2025Learn More -


What Is Brain Microdialysis and Its Application in PK Studies for CNS Drugs
ArticlesSep 26, 2025Learn More -


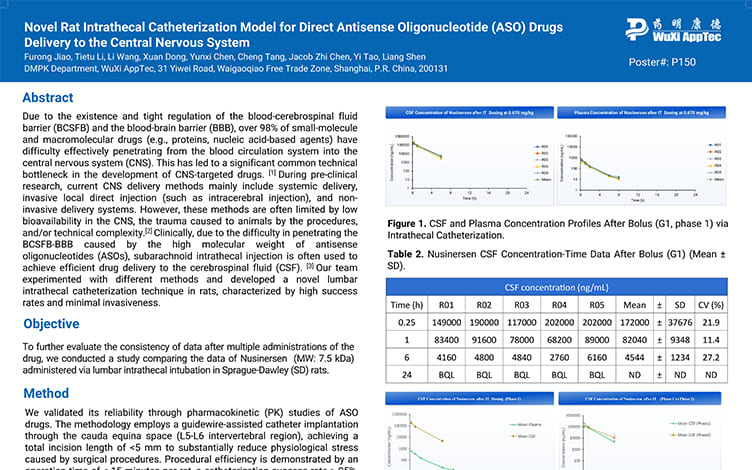
Novel Rat Intrathecal Catheterization Model for Direct Antisense Oligonucleotide (ASO) Drugs Delivery to the Central Nervous System
PostersAug 28, 2025Learn More -


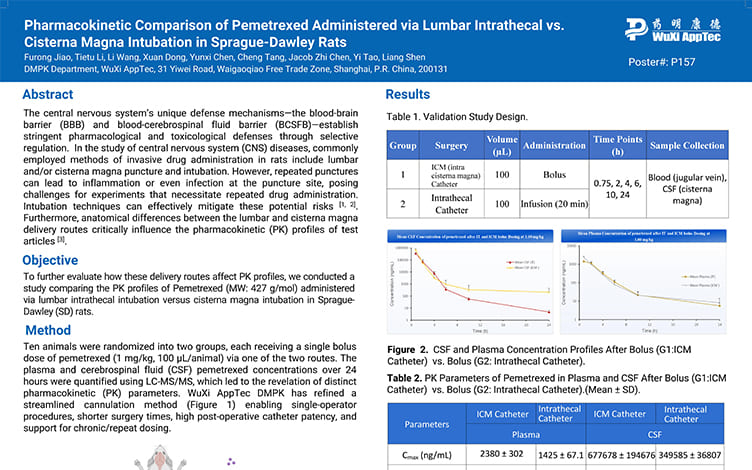
Pharmacokinetic Comparison of Pemetrexed Administered via Lumbar Intrathecal vs.Cisterna Magna Intubation in Sprague-Dawley Rats
PostersJun 12, 2025Learn More -


Effective Intrathecal Injection/Catheterization in Rats for CNS Drugs: Challenges and Strategies
ArticlesJun 12, 2025Learn More -


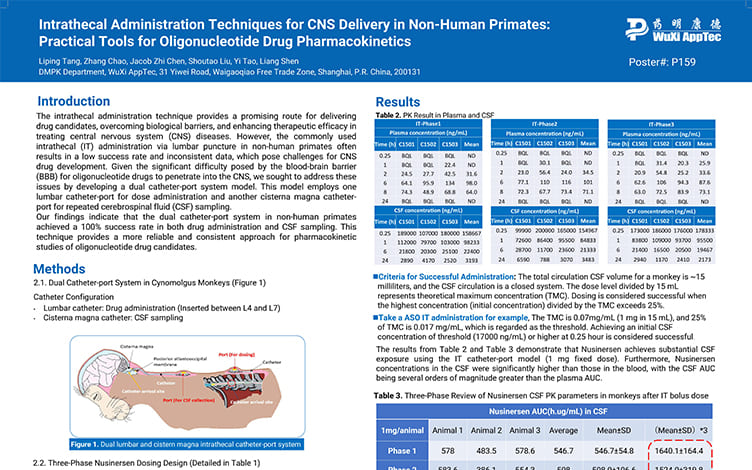
Intrathecal Administration Techniques for CNS Delivery in Non-Human Primates:Practical Tools for Oligonucleotide Drug Pharmacokinetics
PostersJun 05, 2025Learn More -


Crossing the Blood-Brain Barrier for Central Nervous System Drugs: Challenges and Evaluation Strategies
BlogsJun 05, 2025Learn More -


Intrathecal Administration: Breaking Barriers for Central Nervous System Drugs
VideosMay 28, 2025Learn More -


Navigating Complexities in Oligonucleotides: DMPK Strategies and CNS-Targeting Solutions
WebinarsFeb 20, 2025Learn More -


Central Nervous System (CNS) Drugs DMPK Services
BrochuresDec 25, 2024Learn More -


4 Best Practices for Intrathecal Administration in CNS Drug Development
BlogsNov 12, 2024Learn More -


Intrathecal Antisense Oligonucleotides: PK and Strategy
ArticlesJul 04, 2024Learn More
References
- 1.
Darvesh AS, Carroll RT, Geldenhuys WJ, et al. In vivo brain microdialysis: advances in neuropsychopharmacology and drug discovery. Expert Opinion on Drug Discovery. 2011 Feb;6(2):109-127.
- 2.
Sauernheimer C, Williams KM, et al, J Pharmacol Toxicol Methods. 1994 Nov;32(3):149-54.
Stay Connected
Keep up with the latest news and insights.