-
Overview
-
Study Models and Platforms
-
Experience
-
Study Strategies and Assays
-
Case Study
-
FAQs
-
Related Resources
-
Related Services
Overview
WuXi AppTec DMPK offers comprehensive pharmacokinetic research services for peptide drugs to assist you in better optimizing the efficacy and safety of your drugs. Peptide drugs hold significant potential in treating various diseases, but they also present challenges such as low oral bioavailability, poor stability, and a short half-life. Our services aim to provide solutions for these issues. With abundant animal and experimental resources, high-throughput experimental platforms, and cutting-edge equipment, we can provide high-quality data rapidly. Our experienced team can offer customized research strategies, experimental designs, and result interpretations, empowering clients to advance their projects swiftly and effectively.
Learn More


Study Models and Platforms
-
In Vitro Research Platform
Peptide drugs are prone to degradation and metabolism. WuXi AppTec DMPK can carry out stability studies in various matrices such as whole blood, plasma, lysosomes, cathepsin B, liver/kidney S9, and homogenates. In protein binding studies, the use of ultracentrifugation method can reduce the impact of instability and high adsorption.

-
In Vivo Research Platform
WuXi AppTec's DMPK can conduct in vivo PK, excretion, and tissue distribution studies of peptides in various species including mice, rats, dogs, monkeys, rabbits, and mini pigs. We offer solutions for stability and adsorption issues during in vivo sample processing and achieve good separation and low quantitative limits through high-end analytical instruments.

-
Metabolite Identification Platform
Based on HPLC coupled with high-resolution mass spectrometry, combined with various data collection modes, data processing techniques, and metabolite identification software, we can quickly and accurately analyze and identify metabolites of peptides (linear peptides, monocyclic peptides, or polycyclic peptides, etc.) in in vitro and in vivo samples. This provides references for early screening of peptide compounds, IND applications, and subsequent studies of clinical metabolic safety.

-
Immunogenicity Testing (anti-drug antibodies) Platform
Peptide drugs have significantly lower molecular weights than large protein molecules, are cleared faster in the body, and typically have lower immunogenicity. However, additional side chains or modifications may increase immunogenicity risk, and impurities present during peptide synthesis could also lead to a stronger immunogenic response. WuXi AppTec's DMPK has ELISA, MSD, and other LBA platforms to detect immunogenicity (anti-drug antibodies). We usually use the MSD bridging method to measure total ADA, with peptide drugs separately labeled with biotin and ruthenium as capture and detection agents.

-
Radiolabeled Isotope Synthesis, Administration, and Detection Platform
Radiolabeling technology provides a powerful tool for in vivo ADME studies of peptides. WuXi DMPK has the capability to synthesize radiolabeled peptides, selecting the appropriate labeling isotopes and sites based on peptide characteristics. We can also conduct PK, excretion, tissue distribution (including QWBA), and metabolite identification studies of peptide drugs in various species such as mice, rats, dogs, and monkeys using C14 and H3 radiolabeling. With years of experience in peptide research and a track record of NMPA and FDA applications for radiolabeled in vivo ADME, we support global clients in rapidly advancing their peptide drug development process.
Experience
-
15+
Years’ experience in peptide project research
-
10000+
The screening and evaluation of peptide molecules
-
10+
IND applications
-
160+
Global clients of peptide
Study Strategies and Assays
-
Early screening stage
Mainly investigating the in vitro properties (including plasma stability, metabolic stability, permeation, and protein binding), and guide molecular structure optimization. The interactions and enzyme phenotyping of peptides with low molecular weight are evaluated as well.
PCC stage
This stage focuses on the metabolic differences of species and the in vivo PK study of peptides. It aims to select the appropriate species for relevant research by considering both pharmacodynamics and toxicology. The study of various delivery carriers makes it possible to achieve the desired in vivo exposure characteristics.
IND stage
In this stage, single-dose escalation and repeated administration of PK in animals will be studied. The excretion pathway (mass balance), tissue distribution, and in vivo metabolite identification also need to be evaluated.
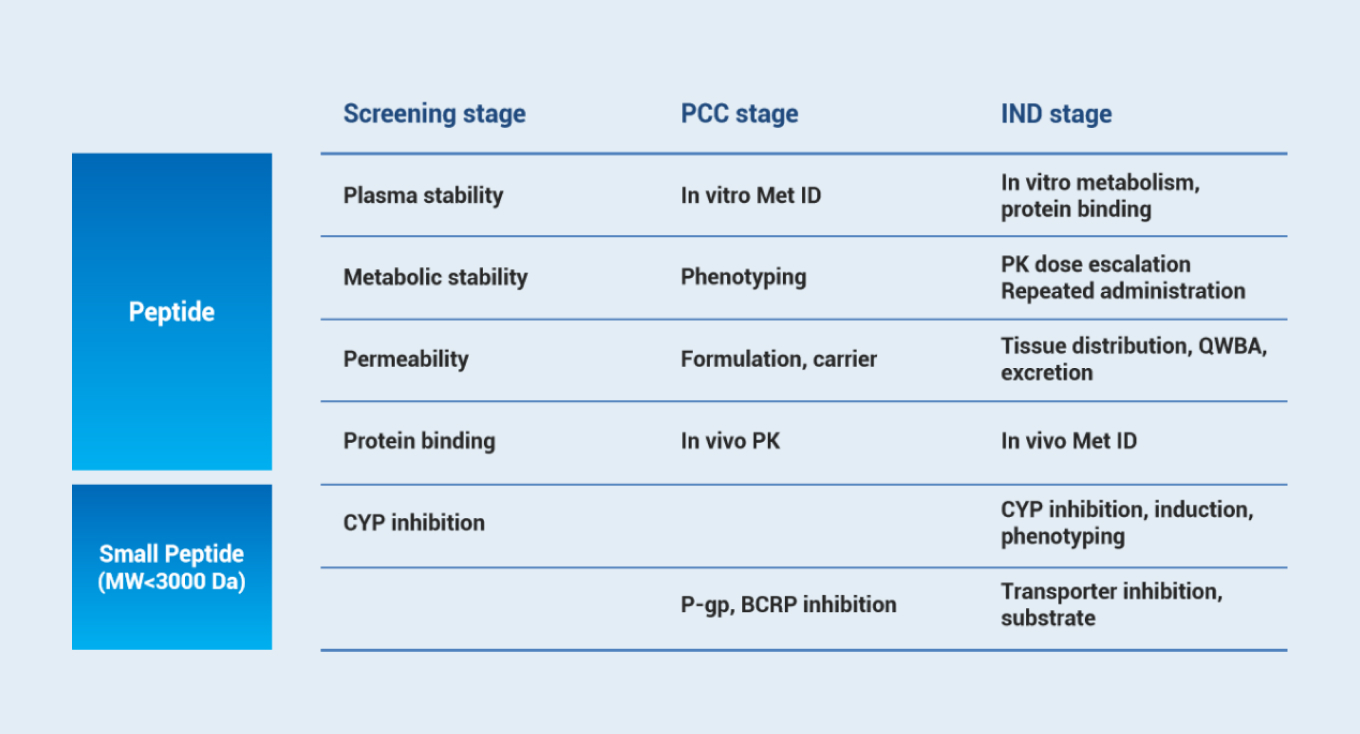
Stage | Screening | Preclinical Candidate | IND |
ADME |
|
|
|
DDI | Peptides with low molecular weight:
| Peptides with low molecular weight:
| Peptides with low molecular weight:
|
PK |
|
|
|
Case Study
-
Bioanalysis of Commercial Compound A by LC-MS/MS
Background
As a synthetic product of Exendin-4, Commercial Compound A consists of 39 amino acids. In this case, the PK in rats was studied. Since the administration dosage designed in this experiment was quite low, the plasma concentration was predicated to be low accordingly, so the detection sensitivity was required to be high (20 ~ 30 pg/mL). In addition, blood biochemical analysis was also required for this project, leaving a miniscule sample volume for PK detection. This posed a significant challenge to method development.
Problems and Solutions
Using special low-adsorption consumables and reagents, the amount of sample adsorbed by the container was greatly reduced.
As for the non-specific binding or co-precipitation between the sample and protein, the sample was alkalinized to improve the recovery rate.
Solid phase extraction (SPE) was adopted to enrich and purify the plasma sample, which eliminated the endogenous interference and further improved the recovery rate.
The lower limit of quantitation (LLOQ) of this procedure reached ~ 20.9 pg/mL. This result was comparable to that of the RIA procedure reported in the literature.
Advantages
Low cost (no need to purchase commercial kits)
High throughput (automated workstation for 96-well plates)
Small sample amount (takes only 50-100 µl of a sample, unlike other procedures that require samples to be hundreds of microliters of a sample)
High specificity
Learn More
-


Fig. (a). LC-MS spectrum of single blank sample
-


Fig. (b). LC-MS spectrum of LLOQ (20.9 pg/mL)
-
FAQs
-
What are peptide drugs?
Peptides are compounds formed by linking α-amino acids together through peptide bonds. Oligopeptides consist of 2 to 20 amino acids, while polypeptides contain more. The FDA defines a "protein" as "any α-amino acid polymer with a specific sequence and a size greater than 40 amino acids", which falls under the Biological License Application (BLA). Therefore, the development of peptide drugs with 40 or fewer amino acids can still refer to the regulatory requirements for small molecules. These drugs have many advantages due to their unique chemical structure and functionality, such as high specificity, few side effects, and strong biological activity.
-
Are peptide drugs cleared fast in the body?
Peptides have relatively short half-lives in the body because they are easily broken down by proteolytic enzymes or quickly excreted by the kidneys. For instance, the natural GLP-1 has a half-life of about 0.9 minutes in the body. However, some designed peptide drugs have longer half-lives in the body. For example, by altering the chemical structure of peptides (such as cyclic peptides), or binding them with other molecules (such as polyethylene glycol), or linking ligands to enhance binding with albumin, their clearance rate in the body can be slowed down.
-
How to select animal species for in vivo PK study of peptide drugs?
PK studies usually need be carried out in both rodent and non-rodent animal species. Species selection can be based on pharmacological, metabolic, toxicological, and other mechanisms. For example, monkeys are generally chosen for PK experiments of GLP-1 receptor agonist drugs because monkeys' endocrine systems and GLP-1 receptors are similar to those of humans. Some peptide drugs can select species that have similar metabolic characteristics to humans.
-
Are immunogenicity studies necessary for peptide drugs?
Peptide drugs, like proteins, may be seen as xenobiotic or antigens by the body, triggering an immune response and possibly generating anti-drug antibodies. However, the risk of immunogenicity of peptides is typically lower than that of protein drugs and is related to the dose and dosage regimen. Small peptide drugs, low doses, and single administration may have a lower incidence of immunogenicity. The need for immunogenicity studies can be evaluated during the clinical phase.
-
Are drug interaction studies necessary for peptide drugs?
Peptide drugs, falling between large and small molecules, currently have no specific guidance for drug interaction studies. According to some expert opinions, peptide drugs with a molecular weight of less than 2000 Da are recommended for drug interaction studies. Larger peptides are generally believed to have no direct interaction with CYP enzymes or transporters.
-
Are radiolabeled studies necessary for peptide drugs?
Peptide drugs have numerous metabolites, and the proportion of the parent drug's exposure in the body is relatively small. Conducting metabolism, tissue distribution, and excretion studies using radiolabeled isotopes can provide more comprehensive results.
-
How are peptides used in medicine?
Peptide therapeutics are widely used in the pharmaceutical field due to their high activity, specificity, and low toxicity. The approved indications for marketed peptide drugs primarily include endocrinology, oncology, and metabolic-related diseases. Peptides can also serve as targeting moieties, conjugated with payloads to form peptide-drug conjugates (PDC), enhancing therapeutic efficacy while reducing side effects. Beyond therapeutic drugs, peptide vaccines and targeted peptide probes are employed in disease prevention and diagnostics. Current research directions in peptides include oral peptides, cell-penetrating peptides, and antimicrobial peptides. It is anticipated that more peptide drugs will provide novel therapeutic approaches in the future.
Related Resources




-


Software-aided Detection and Structural Characterization of Cyclic Peptide Metabolites in Biological Matrix by High-resolution Mass Spectrometry
PublicationsJan 09, 2026Learn More -


GLP-1 Peptide Drug Radiolabeling and ADME Study Strategies
ArticlesDec 24, 2025Learn More -


Decoding Peptide ADME Challenges: Integrated Radiolabeled Synthesis & ADME Platform - DMPK Frontiers
VideosOct 24, 2025Learn More -


In Vitro Metabolic Stability of Peptide Drugs Across Different Tissues
ArticlesSep 30, 2025Learn More -


Biomimetic Chromatography Method to Estimate Human Plasma Protein Binding for Peptides
PostersSep 19, 2025Learn More -


Establish a High-throughput Exposed Polar Surface Area (EPSA) Screening Platform
PostersSep 04, 2025Learn More -


Evaluating Oral Peptides' Gastrointestinal Absorption: Methods and Applications
ArticlesAug 28, 2025Learn More -


Advancing Novel Therapeutics with Innovative DMPK Strategies in Metabolism and Bioanalysis
WebinarsAug 22, 2025Learn More -


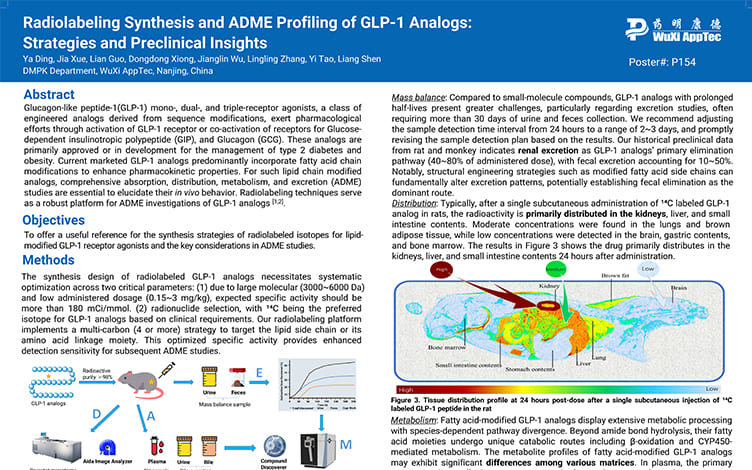
Radiolabeling Synthesis and ADME Profiling of GLP-1 Analogs: Strategies and Preclinical Insights
PostersAug 22, 2025Learn More -


Solving the Peptide Puzzle: Strategies for Smarter Development
WebinarsJul 24, 2025Learn More -


An Integrated Radiolabeling and ADME Platform for Peptide Drugs
WebinarsJul 17, 2025Learn More -


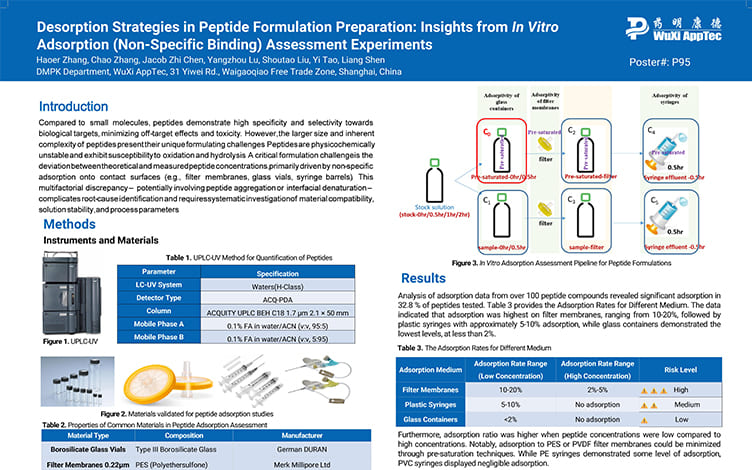
Desorption Strategies in Peptide Formulation Preparation: Insights from In Vitro Adsorption (Non-Specific Binding) Assessment Experiments
PostersJul 17, 2025Learn More -


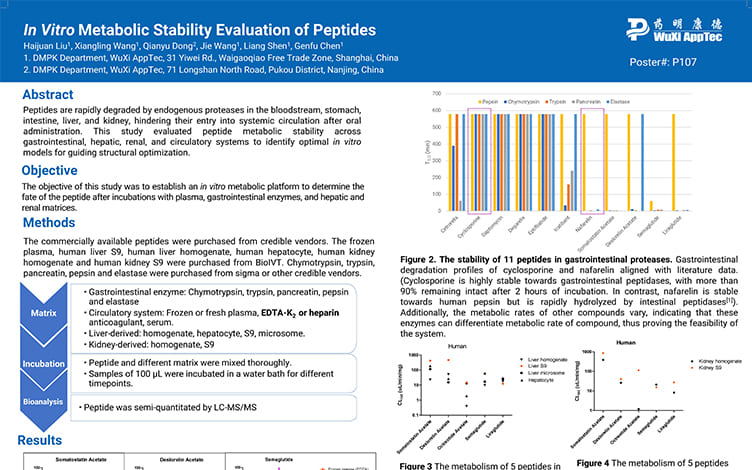
In Vitro Metabolic Stability Evaluation of Peptides
PostersJul 16, 2025Learn More -


A Novel Validated LC-MS/MS Method for the Determination of Survodutide in Mouse Liver
PostersJul 04, 2025Learn More -


Cyclic Peptides: FDA-Approved Drugs and Their Oral Bioavailability and Metabolic Stability Tactics
ArticlesMay 08, 2025Learn More -


Enhancing Permeability Through Exposed Polar Surface Area (EPSA) for Beyond Rule of Five (bRo5) Drug Candidates
ArticlesApr 10, 2025Learn More -


Unlocking Precision in Peptide Metabolite Identification for Next-Gen Drug Development
ArticlesMar 19, 2025Learn More -


Webinar Q and A | Optimizing DMPK Strategy for Peptide Drugs
BlogsJan 13, 2025Learn More -


Metabolite Identification in Peptide Drugs and Its Challenges
ArticlesDec 02, 2024Learn More -


Optimizing DMPK Strategy for Peptide Drugs
WebinarsNov 21, 2024Learn More -


Exploring GLP-1 Receptor Agonists (GLP-1 RAs): R&D Advances and Tackling Bioanalytical Challenges
ArticlesNov 01, 2024Learn More -


DMPK Research of Cyclic Peptides
WebinarsOct 29, 2024Learn More -


Decoding DMPK Profiles of 37 FDA-Approved Peptides: Insights and Radiolabeling Considerations
BlogsOct 18, 2024Learn More -


A Rapid Liquid Chromatography-Mass Spectrometry Quantitation of GLP-1 Receptor Agonist in Rat Plasma
PostersJul 26, 2024Learn More -


Preclinical Drug Development Testing for Peptide
BrochuresMay 18, 2023Learn More
Stay Connected
Keep up with the latest news and insights.