-
Overview
-
Services
-
Featured Strengths
-
FAQs
-
Related Resources
-
Related Services
Overview
Our in vitro ADME services team offers a very wide services in the field of Physicochemical Properties, Permeability, Metabolic Stability, Drug-Drug Interaction, Transporter, and Protein Binding and Partitioning. We also have a variety of advanced automated liquid workstations and LC-MS/MS analysis technologies to further improve the assays' speed, quality, and throughput. More new modalities with complex structures have emerged from the recent R&D pipelines, including Proteolysis-Targeting Chimeras (PROTACs*), oligonucleotides, and antibody-conjugated drugs (ADCs). There are many challenges in the in vitro ADME study of these new modalities. WuXi AppTec DMPK continuously expand the platforms to support the ADME studies of these new modalities.
Learn More

Services
Typically, in the Lead Finding (LF) stage, compounds' solubility, lipophilicity, permeability, and microsomal stability are determined first. The obtained data are used to build the structure-property relationship of compounds, screen chemical structure categories, and confirm the priority of multiple structural backbones. At the Lead Optimization (LO) and Pre-Clinical Candidate (PCC) stages, comprehensive in vitro ADME studies are usually required. These include multi-species metabolic stability, plasma protein binding, transporters-related drug interaction, and inhibition of the drug-metabolizing enzymes combined with animal PK data to predict human PK performance. In the Investigational New Drug (IND) stage, a comprehensive evaluation of in vitro ADME properties is required and conducted per the requirements of drug registration authorities. It should be noted that the in vitro ADME screening study is usually not designed for regulatory submission purpose.
Learn More

-


Learn More
Physicochemical Property
Solubility (KS, TS), pKa, logD, logP, and PSA
-


Learn More
Permeability
PAMPA, Caco-2, MDCK I, MDR1-MDCK I, MDCK II, and MDR1-MDCK II
Ex vivo Franz cell-based dermal penetration assay
-


Learn More
Whole Blood/Plasma Distribution and Protein Binding
B/P ratio
Dialysis, ultracentrifugation, ultrafiltration, flux dialysis, competition dialysis, etc
-


Learn More
Drug-drug Interaction
Metabolism-mediated drug interactions
Transporter-mediated drug interactions
-


Learn More
Metabolism
Matrix stability (Tissue, plasma, SGF/SIF, buffer, whole blood, GSH reaction, etc.)
Metabolic stability (Microsomes, S9, hepatocytes, cytosol, lysosome, mitochondria, recombinase, etc.)
Featured Strengths
-


High-throughput and comprehensive service types
We can provide various ADME services from screening to IND application, with more than 100 types of assays and one-stop service to the clients. Multiple automation can ensure continuous improvement of throughput.
-


Fast data delivery
Based on solid technology, optimized operation, and improved throughput, we can save time and cost for the clients. For most screening assays, no more than 5 working days are needed from compound receipt to report submission.
-


High-quality data
The application of automation can reduce human errors and improve data quality. A complete set of in vitro ADME services can be provided to meet the submission requirements from the FDA, EMA, and NMPA for new drugs. Currently, the IND applications of hundreds of drug candidates have been completed, and all of them have passed the on-site audit by the regulatory authority.
-


Experienced researchers
The core team has more than 15 years of ADME research experience, which can provide the most effective data in the shortest time, save resources, and accelerate the development process.
FAQs
-
What is In Vitro ADME test?
In vitro comes from the Latin term "in glass". It refers to studies that are performed outside of a living organism in a controlled environment, such as a test tube or petri dish. In vitro studies are often contrasted with in vivo ("within the living") studies, which are done in a whole living organism.
The in vitro testing is ideal for testing a potential drug compound with well-controlled testing conditions at an early stage. In vitro testing requires less resources and tends to be much quicker than in vivo models. In vitro testing helps drug developers understand if the drug candidate is suitable to move forward with further investigations.
-
What does In Vitro ADME test study?
In vitro ADME studies absorption (A), distribution (D), metabolism (M), and excretion (E) properties of the drug candidates in a panel of assays outside of a living organism. The deions of each keyword are as follows.
Absorption: Describes the compound’s ability to pass through physiological barriers such as the intestines, skin, etc.
Distribution: Describes how the compound is distributed throughout the body or tissue once it has been taken into the body.
Metabolism: Describes how the body breaks down the compound in organs such as liver, kidney, skin or the gut.
Excretion: Describes how the body removes the compound.
Our platform can also provide drug-drug interaction evaluation.
-
Why is in vitro ADME testing important?
Reduce R&D costs and risks
Quickly assess the ADME potential of candidate compounds early in development to eliminate unsuitable ones as soon as possible.
Increase screening efficiency
In vitro models (e.g., Caco-2 cells for absorption, liver microsomes for metabolism) can process large numbers of compounds rapidly, testing the ADME properties of hundreds to thousands of candidates, thereby shortening the time to preclinical research.
Guide molecular structure optimization
Identify deficiencies in candidate compounds' absorption and metabolism (e.g., poor permeability, enzyme degradation) and guide medicinal chemistry teams to modify the molecular structure, improving drug-likeness.
Reduce reliance on animal testing
In vitro testing reduces the need for live animals, complies with the 3R principles (Replace, Reduce, Refine), and reveals species-specific physiological differences, providing valuable insights for interpreting in vivo data while meeting international ethical standards.
Provide preclinical and clinical trial guidance
In vitro ADME data can be used to predict drug behavior in humans (e.g., bioavailability, half-life), inform dosage design and administration routes for animal studies, and help anticipate potential issues in clinical trials (e.g., drug interactions,- individual variability).
Related Resources




-


What is Alpha-1 Acid Glycoprotein (AAG) and Its Role in Drug Binding for Pharmacokinetics and Pharmacodynamics Studies
ArticlesJan 09, 2026Learn More -


DDIM vs. TDI in CYP Enzyme Inhibition Studies: Can Results from a CYP Direct Inhibition Assay be Used to Predict Time-Dependent Inhibition Risk?
ArticlesDec 19, 2025Learn More -


Tackling the CNS Drug Challenges with DMPK Toolkit
WebinarsDec 05, 2025Learn More -


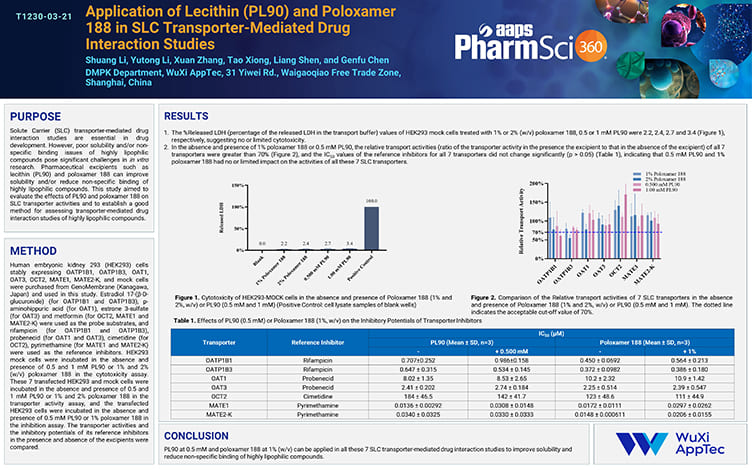
Application of Lecithin (PL90) and Poloxamer 188 in SLC Transporter-Mediated Drug Interaction Studies
PostersNov 21, 2025Learn More -


Pregnane X Receptor (PXR) in Drug Metabolism: Mechanism, Role, and HepG2-Based Screening
BlogsNov 07, 2025Learn More -


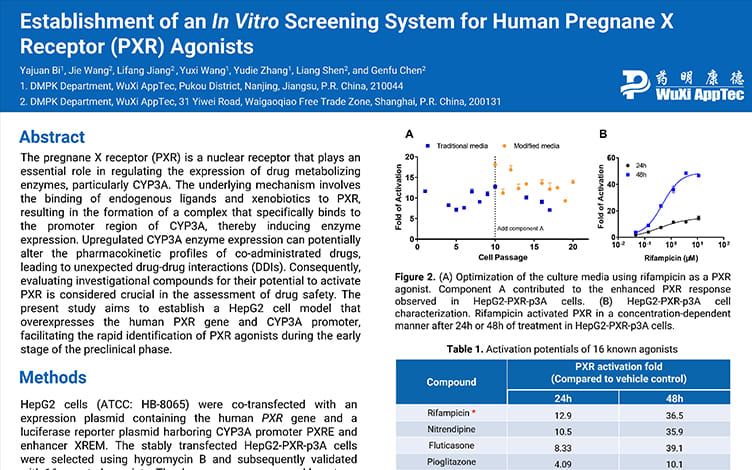
Establishment of an In Vitro Screening System for Human Pregnane X Receptor (PXR) Agonists
PostersNov 07, 2025Learn More -


Overcoming Challenges in Oligonucleotide Metabolism with Innovative Solutions -WuXi AppTec DMPK TechTalk
VideosNov 07, 2025Learn More -


Evaluating Cytochrome P450 (CYP) Enzyme Inhibition: Hepatocytes vs. Liver Microsomes
BlogsOct 31, 2025Learn More -


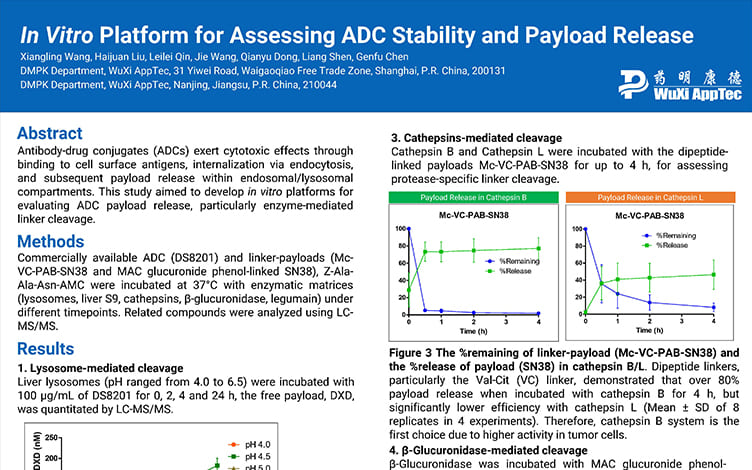
In Vitro Platform for Assessing ADC Stability and Payload Release
PostersOct 31, 2025Learn More -


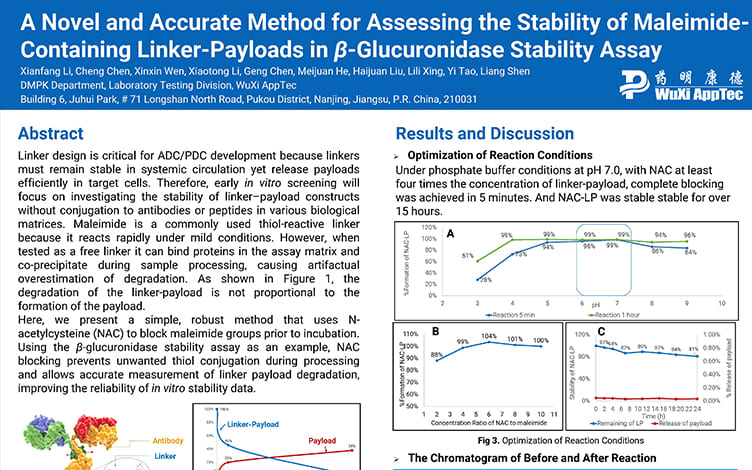
A Novel and Accurate Method for Assessing the Stability of Maleimide-Containing Linker-Payloads in the β-Glucuronidase Stability Assay
PostersOct 12, 2025Learn More -


Establishment of a Novel Funnel Model for Evaluating Blood-Brain Barrier Penetration In Vitro
PostersSep 30, 2025Learn More -


In Vitro Metabolic Stability of Peptide Drugs Across Different Tissues
ArticlesSep 30, 2025Learn More -


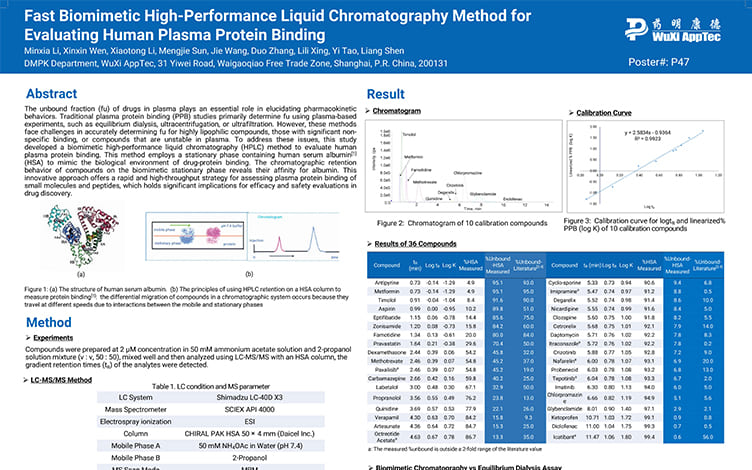
Fast Biomimetic High-Performance Liquid Chromatography Method for Evaluating Human Plasma Protein Binding
PostersSep 30, 2025Learn More -


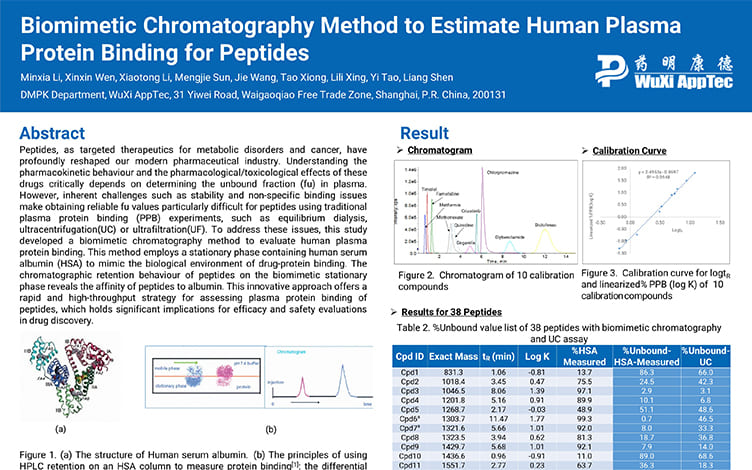
Biomimetic Chromatography Method to Estimate Human Plasma Protein Binding for Peptides
PostersSep 19, 2025Learn More -


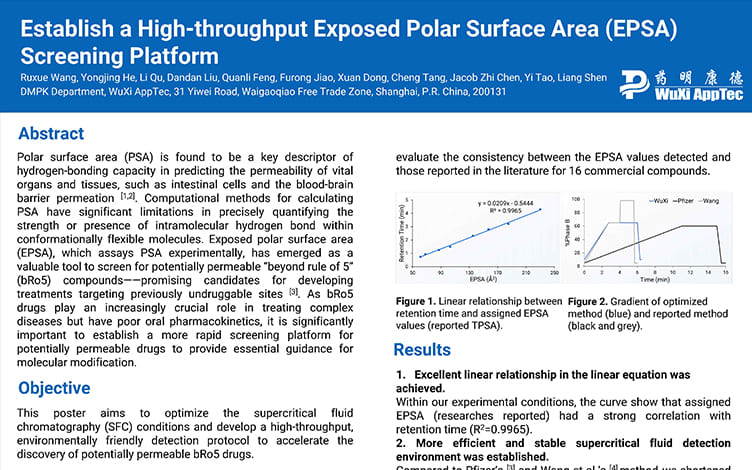
Establish a High-throughput Exposed Polar Surface Area (EPSA) Screening Platform
PostersSep 04, 2025Learn More -


WuXi AppTec DMPK In Vitro ADME – Illuminating Your Drug Discovery Journeys
VideosSep 04, 2025Learn More -


Evaluating Oral Peptides' Gastrointestinal Absorption: Methods and Applications
ArticlesAug 28, 2025Learn More -


Advancing Novel Therapeutics with Innovative DMPK Strategies in Metabolism and Bioanalysis
WebinarsAug 22, 2025Learn More -


Decoding the Key Role of Plasma Protein Binding in Drug-Drug Interactions
ArticlesAug 15, 2025Learn More -


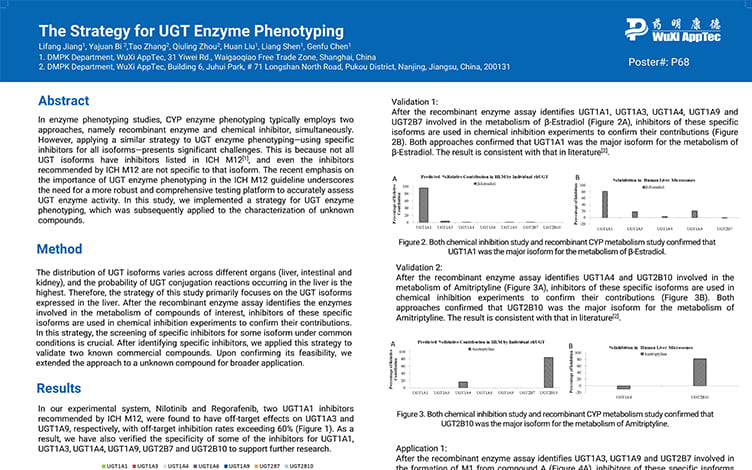
The Strategy for UGT Enzyme Phenotyping
PostersAug 12, 2025Learn More -


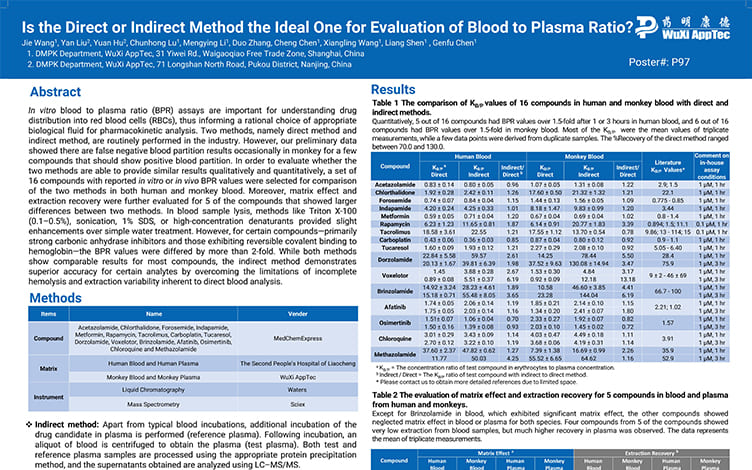
Is the Direct or Indirect Method the Ideal One for Evaluation of Blood to Plasma Ratio?
PostersAug 06, 2025Learn More -


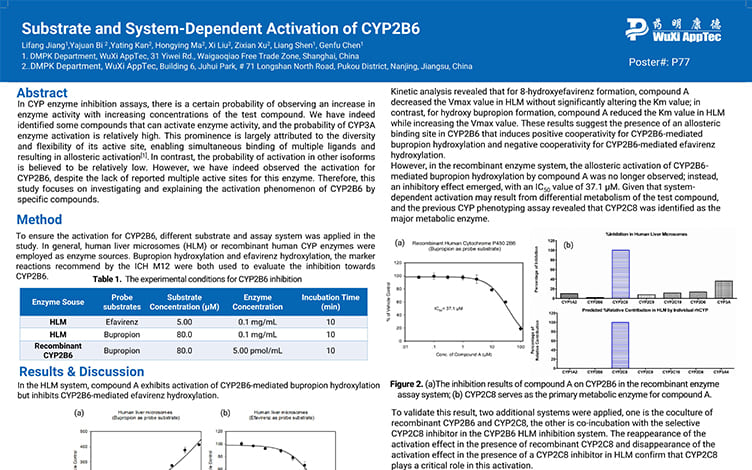
Substrate and System-Dependent Activation of CYP2B6
PostersAug 06, 2025Learn More -


Factsheet- Cytochrome P450 Induction: Relative Induction Score (RIS) Analysis
BrochuresAug 01, 2025Learn More -


More Accurate Prediction of Human Pharmacokinetic Parameters for Small Molecule Compounds: Methods, Models, and Applications
ArticlesAug 01, 2025Learn More -


Antibody-Drug Conjugate (ADC) R&D Innovations and In Vitro ADME Research Considerations
ArticlesJul 24, 2025Learn More -


Development of a High-throughput and Cost-effective Method for Experimental Polar Surface Area Measurement with Ultra-Performance Convergence Chromatography Tandem Mass Spectrometry
PostersJul 24, 2025Learn More -


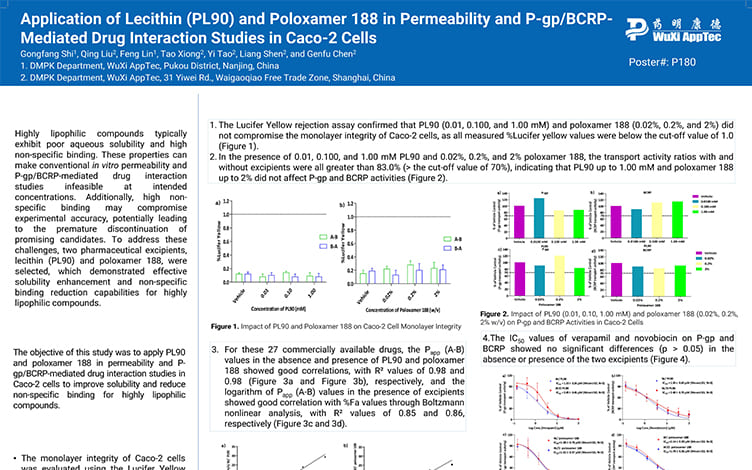
Application of Lecithin(PL90) and Poloxamer 188 in Permeability and P-gp/BCRP-Mediated Drug Interaction Studies in Caco-2 Cells
PostersJul 23, 2025Learn More -


New Book Release: Drug Metabolism and Pharmacokinetics: Frontiers, Strategies, and Applications | WuXi AppTec DMPK
VideosJul 17, 2025Learn More -


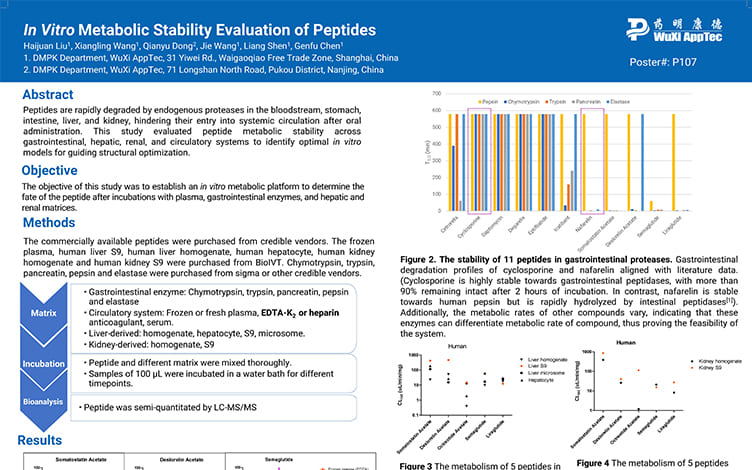
In Vitro Metabolic Stability Evaluation of Peptides
PostersJul 16, 2025Learn More -


Decoding DMPK Frontiers for Novel Therapeutics: WuXi AppTec DMPK’s New Book Release
BlogsJul 10, 2025Learn More -


ICH M12 Guideline: 4 Key Updates and Reshaping Framework for Enzyme-Mediated Drug-Drug Interaction Studies
ArticlesJun 27, 2025Learn More -


In Vitro Metabolism Methods for Oligonucleotides
White PapersJun 20, 2025Learn More -


Drug Metabolism and Pharmacokinetics: Frontiers, Strategies, and Applications
PublicationsJun 13, 2025Learn More -


Advancing In Vitro Metabolic Models and Metabolite Identification for Oligonucleotide Therapeutics
WebinarsJun 05, 2025Learn More -


High-Performance, Professional, Innovative: WuXi AppTec DMPK Accelerates Breakthroughs
VideosMay 22, 2025Learn More -


Cyclic Peptides: FDA-Approved Drugs and Their Oral Bioavailability and Metabolic Stability Tactics
ArticlesMay 08, 2025Learn More -


In Vitro ADME Biosafety Level 2 Labs at WuXi AppTec DMPK
VideosApr 18, 2025Learn More -


How Biosafety Level 2 Labs Ensure Safe and Compliant In Vitro ADME Studies
BlogsApr 17, 2025Learn More -


Covalent Drugs DMPK Services
BrochuresApr 10, 2025Learn More -


Enhancing Permeability Through Exposed Polar Surface Area (EPSA) for Beyond Rule of Five (bRo5) Drug Candidates
ArticlesApr 10, 2025Learn More -


FAQs on Oligonucleotide DMPK Studies Stability, Distribution, Administration, and Bioanalysis
BlogsApr 03, 2025Learn More -


Methods and Strategies for In Vitro Metabolism Studies of Oligonucleotides
ArticlesJan 08, 2025Learn More -


WuXi AppTec DMPK: Your Trustworthy Partner
VideosDec 27, 2024Learn More -


CYP17A1/19A1 Inhibition Services
BrochuresDec 12, 2024Learn More -


A Rapid Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Method for Quantifying the Metabolites of Seven Major Probe Substrates in Human Liver Microsomes
PostersDec 11, 2024Learn More -


Advancing Antibody-Drug Conjugates (ADCs) with Novel Payloads and Their DMPK Considerations
ArticlesDec 10, 2024Learn More -


Impact of Bovine Serum Albumin on In Vitro Permeability and ABC Transporter Mediated Drug Interaction Evaluation
PostersDec 03, 2024Learn More -


The Determinants in Evaluating siRNA Plasma Protein Binding Using Agarose Gel Electrophoretic Mobility Shift Assay (EMSA)
PostersDec 03, 2024Learn More -


A Sensitive and Efficient LC-MS/MS Method for Creatinine-d5 Analysis in In Vitro OCT2 Inhibition Assay
PostersNov 28, 2024Learn More -


Does the Flux Competition Method Add Significant Value in Species Difference Protein Binding Evaluation?
PostersNov 21, 2024Learn More
Stay Connected
Keep up with the latest news and insights.