-
Overview
-
Methods
-
Case Study
-
FAQs
-
Related Resources
-
Related Services
Overview
IMHBs correlate with the increase of membrane permeability, thus, the identification of compounds potential to form intramolecular hydrogen bonds and hide polarity and improving passive permeability are important factors for drug design. Traditional membrane permeability measurement methods, such as cell model-based drug screening or PAMPA (parallel artificial membrane permeation assay), have low applicability to macromolecules. Since main obstacle of peptide permeability is polarity, the EPSA (exposed polar surface area) technique is a suitable tool for this kind of assay. WuXi AppTec DMPK uses well recognized SFC chromatographic method to detect EPSA. It can achieve high sensitivity, accuracy, great reproducibility of the results, and rapid prediction of cell permeability. In addition, it is an efficient supplement for the existing cell permeability measurement and prediction methods and could provide a reference for pharmacokinetic property optimization.
Learn More


Methods
-
Theoretical Concentration
100 μM
Mobile phase
20 mM Ammonium Formate in MeOH/CO2
Sample Volume Required
20 μL of DMSO stock solution at 10 mM sample concentration
Analytical Method
UPCC-PDA/UPCC-MS/MS
Turnaround Time
5 working days
Case Study
-
Polarity is an essential physicochemical property of molecules for impacting passive permeability. The DMPK team has successfully established the method of EPSA detection, a high throughput chromatographic method with SFC technique, which provides an important index for identifying hidden IMHBs in molecules, assesses the exposure polarity, and predicts molecular permeability.
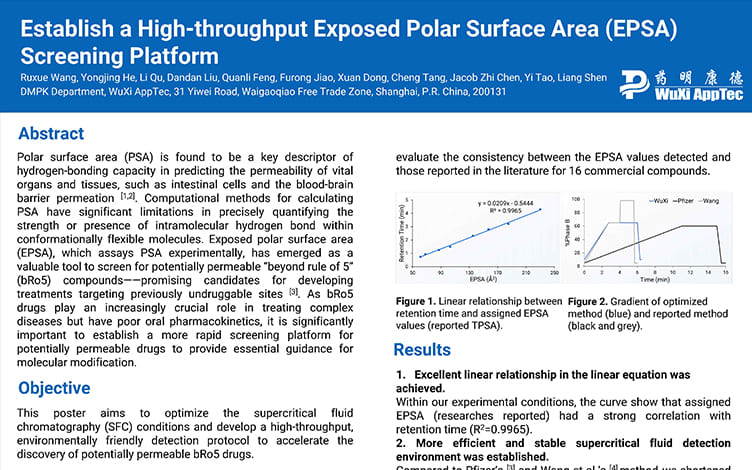
Eight compounds are selected as calibration standards based on their retention times and EPSA values. The linear relation established is shown in Figure 1.
Validation data of the correlation with literature based on EPSA and Bland Altman analysis for 14 commercially available compounds is shown in Figure 2 and Figure 3.
-


Figure 1
-


Figure 2
-


Figure 3
-
FAQs
-
What are the significant advantages of EPSA compared to TPSA (topological polar surface area)?
TPSA is a theoretical computational method by acquiring the surface area of polar atoms with computer software platforms. It evaluates molecular polarity and indicates the spatial distribution of polar atoms rather than their actual exposure. While EPSA is an experimental measurement method that analyzes the exposed polar regions on the molecular surface, which may interact with solvents or other molecules. Both methods can predict the solubility, permeability, and bioavailability of drugs. However, EPSA values with SFC technique could evaluate the polarity and the consequent H-bond capability of the average conformations that analytes adopt in the inner hydrophobic core of the lipid bilayers. Thus, EPSA is a better permeability prediction method in the early stages of drug development.
-
Why is the SFC system better used for detecting EPSA values?
In SFC the retention of an analyte toward the solid phase is predominantly driven by polarity, and this behavior enables identification of IMHBs between matched pairs of molecules through retention time analysis. Compared to using a mobile phase composed of water and organic solvents miscible with water, scCO2 as the mobile phase can avoid districting any IMHBs within the target compounds.
In addition, The EPSA determination with SFC, is faster, more robust, and can be applied for compound displaying high polarity by using an organic modifier as mobile phase ingredient.
Furthermore, the chromatographic retention measurements in SFC systems are performed in an anisotropic environment, which means that the interactions between molecules are heavily influenced by the spatial orientation and topological conformer distribution. This anisotropy is more akin to the characteristics of biological membranes. Therefore, the EPSA with the SFC system is considered to be more predictive of the biological activity or other properties of compounds.
-
Can EPSA be accurately determined if the compound has no UV absorption?
Both PDA method and Mass Spectrometry method have been established, and when the compound lacks UV absorption, UPLC-MS/MS analysis with MRM mode is employed.
-
For CNS and cyclic peptide drugs, does EPSA have a referenced threshold?
The larger the PSA, the greater the polarity, and the more difficult for the compound to permeate membranes. Literatures indicates that Molecules with a PSA of greater than 140 angstroms squared are usually believed to be poor at permeating cell membranes. For molecules to penetrate the BBB (and thus acting on receptors in the central nervous system), PSA should be less than 60 angstroms squared. For a peptide with a measured EPSA above 100 generally will not have significant passive permeability. Therefore, in order to not miss potentially permeable peptides, it is suggested that EPSA could be used as a filter, with a cutoff value of 100.
-
As an index of permeability, is the retention of EPSA related to lipophilicity (Log P)?
EPSA retention is based on polar interactions, independent of molecular weight and lipophilicity (Log P).
Related Resources




-


Establish a High-throughput Exposed Polar Surface Area (EPSA) Screening Platform
PostersSep 04, 2025Learn More -


Development of a High-throughput and Cost-effective Method for Experimental Polar Surface Area Measurement with Ultra-Performance Convergence Chromatography Tandem Mass Spectrometry
PostersJul 24, 2025Learn More -


Enhancing Permeability Through Exposed Polar Surface Area (EPSA) for Beyond Rule of Five (bRo5) Drug Candidates
ArticlesApr 10, 2025Learn More -


Rapid Determination of Lipophilicity: Exploration and Establishment of Reversed-Phase Liquid Chromatography (RPLC) Methods
PostersNov 05, 2024Learn More -


Ensuring drug product integrity: The crucial role of stability testing
BlogsOct 27, 2024Learn More -


How to Evaluate Lipophilicity Rapidly? The Significance of Reversed-Phase Liquid Chromatography (RP-HPLC)
BlogsDec 19, 2023Learn More -


Rapid Determination of Lipophilicity: Establishment and Application of Reversed-Phase Liquid Chromatography (RP-HPLC)
ArticlesNov 30, 2023Learn More -


Focusing on PROTAC Permeability and Solubility Improving the Oral Availability
BlogsJul 07, 2023Learn More -


Research on PROTAC Druggability: Solubility and Permeability
ArticlesJun 30, 2023Learn More
References
- 1.
Goetz, G. H.; Philippe, L.; Shapiro, M. J. EPSA: a novel supercritical fluid chromatography technique enabling the design of permeable cyclic peptides. ACS Med. Chem. Lett. 2014, 5, 1167−1172.
- 2.
Palm K, Luthman K, Ungell AL, Strandlund G, Artursson P; J Pharm Sci. 1996, 85:32–39.
- 3.
Biron, E.; Chatterjee, J.; Ovadia, O.; Langenegger, D.; Brueggen, J.; Hoyer, D.; Schmid, H. A.; Jelinek, R.; Gilon, C.; Hoffman, A.; Kessler, H. Improving Oral Bioavailability of Peptides by Multiple NMethylation: Somatostatin Analogues. Angew. Chem. 2008, 47, 2595− 2599.
- 4.
Palm K, Stenberg P, Luthman K, Artursson P; Pharm Res. 1997, 14:568–571.
- 5.
Goetz, G. H.; Farrell, W.; Shalaeva, M.; Sciabola, S.; Anderson,D.; Yan, J.; Philippe, L.; Shapiro, M. J. High Throughput Method for the Indirect Detection of Intramolecular Hydrogen Bonding. J. Med.Chem. 2014, 57, 2920−2929
Stay Connected
Keep up with the latest news and insights.