-
Overview
-
Assays
-
Animal Species
-
Route of Administration
-
Surgical Models
-
Experience
-
Facilities
-
FAQs
-
Related Resources
-
Related Services
Overview
Our team has developed more than 30 routes of administration and more than 10 surgery models based on market needs and provides high-quality rodent in vivo PK services to thousands of customers worldwide. The team has also established many unique technical capabilities, such as intravenous infusion for more than 7 consecutive days. The team has launched many high-quality capabilities for the transdermal PK, respiratory PK, ophthalmology PK, and neuro PK and provided many trouble-shooting solutions for customer studies.
Learn More


Assays
-
Hit to lead
Fast PK:
Rat and mouse PK (oral or intravenous administration), single or cassette dosing
-
Lead optimization
PK study in rats and mice (administered through oral, intravenous, subcutaneous, intramuscular, and intraperitoneal routes).
Single or multiple doses
Exposure studies with different formulation
-
Preclinical candidate (PCC)
Single-dose or multiple-dose experiment following specific administration route
PK experiments with different salt forms or crystal forms
PK experiments with different dosage forms, including bridging studies with clinical formulations
Maximum tolerated dose experiment (MTD) and dose escalation experiment
-
Investigational New Drug (IND) application
PK studies of single-dose and single intravenous administration
Oral administration PK studies with high, medium, and low doses
PK studies of medium dose and multiple oral administration
Tissue distribution studies after a single oral administration
Biliary excretion studies with medium dose and single oral administration
Urine and fecal excretion studies with medium dose and oral administration
Identify major metabolites in plasma and excretion
Animal Species
-
Mice
ICR (CD-1)
C57BL/6
BALB/c
FVB
BALB/c Nude
NOD SCID
P-gp/BCRP KO Mouse
-
Rat
Sprague Dawley
Wistar Han
Wistar
Brown Norway
F344(CDF)
Lewis
-
Hamster
LVG Golden Syrian Hamster
-
Guinea Pig
Harley Guinea Pig
Route of Administration
Case Sharing of Administration Route
-
-
Capsule administration
-
Intrathecal injection
-
7-day continuous intravenous infusion
-
Intranasal administration
-
Intratracheal administration
-
Inhalation administration
-
Mini-pump implant
-
Intravitreal injection
-
Intracerebroventricular administration
-
Intravenous bolus injection
-
Intravenous Infusion
-
Oral gavage
-
Intramuscular injection
-
Subcutaneous injection
-
Intradermal injection
-
Intraperitoneal injection
-
Intestinal administration
-
Sublingual administration
-
Transdermal administration
-
Eye drop administration
-
Intra-articular injection
-
Rectal administration
-
Vaginal administration
-
Intravesical administration
-
-
The transdermal drug delivery system (TDDS) has always been an attractive field as it can provide a therapeutic window larger than whole-body delivery. Topical skin formulations are widely used for topical administration, exert local effects, and cause systemic effects through skin absorption. Methods of evaluating topical drug transdermal delivery are divided into in vivo and in vitro 1. Skin absorption is mainly a passive diffusion process. Literature has proven that the data obtained from in vitro studies complying with the appropriate protocol are of great reference values. Compared with traditional in vivo experiments, in vitro methods can measure the drug concentration retained in the skin and the amount that diffused through the skin into the receptor chamber. This method allows the exploration of skin permeability at the early screening stage. No live animals are used in this study, and multiple replicates can be applied at the same or different compounds. This feature is especially suitable for comparing the skin penetration of the same compounds in various in vivo studies complying with the appropriate protocol are of great reference values. Compared with traditional in vitro formulations and screening the transdermal properties of different compounds 2 In vitro, transdermal experiments tested the drug permeability of different batches of drugs using Bama minipigs' skin. The data showed no significant difference in the in vitro permeation characteristics between the test and the reference.
-


24h Permeation Profile using Bama Miniature Pig Skin
Figure 1
-


24h Permeation Profile using Bama Miniature Pig Skin
Figure 2
-


24h Skin Retention of a Drug in Bama Miniature Pigs' Skin
Figure 3
-


Permeation Flux Reached Steady State using
Figure 4
-
Surgical Models
Case Sharing of Surgical Model
-
-
Vascular Cannulation
Carotid
HJugular vein
Femoral artery
Femoral vein
Hepatic portal vein
-
Non-vascular Cannulation
Bile duct
Duodenum
Mesenteric lymph
Cisterna magna cannulation
-
Non-survival Surgery
In situ small intestine perfusion
Isolated liver perfusion
In situ brain perfusion
Nasal lavage
Bronchoalveolar lavage
-
-
In situ single-pass intestinal perfusion (SPIP) model: To date, the most used model for predicting the absorption process in humans is the Caco-2 cell monolayer system. While drug permeability correlates well with drug absorption in humans and the Caco-2 studies, there are many limitations when studying carrier-mediated transport or monitoring intestinal metabolites 3. In situ single-pass intestinal perfusion (SPIP) is the closest experimental method that mimics oral administration and directly measures compound absorption rate. The intestinal structure is similar in both humans and rodents, and there is a strong correlation between effective permeability (Peff) and fraction absorbed (Fa) between these two species (R2 = 0.8 to 0.95). It is easy to control the intestinal environment in SPIP studies, such as drug concentration in the perfusate, perfusate pH, perfusion flow rate, and target intestine perfusion part of the study. More importantly, the SPIP enables simultaneous measurement of the effective permeability (Peff) and flux of drugs appearing in mesenteric blood (Papp) 4, 5. The team can use SPIP models in rodents to assess the intestinal permeability of test compounds and monitor their metabolites.
Group Test Compound Peff from Literature
(×10-6 cm/s)6,7
Peff from
Verification Studies
(×10-6 cm/s)
Average Papp
(×10-6 cm/s)
High
Permeability
Carbamazepine
Propranolol
Metoprolol62-160
41-69
14.3-50.285.60±20.54
59.69±7.31
23.71±0.9234.18±14.47
34.47±6.48
1.51±0.03Medium
Permeability
Ranitidine
Dexamethasone
Atenolol14.7-30
16-24
1.8-1616.84±3.79
17.79±6.39
3.46±1.2013.77±6.64
3.82±0.82
3.15±1.36Low
Permeability
Topotecan
Nadolol1.7-55.8
2.7-5.31.70±2.91
3.52±8.8911.49±1.46
3.22±2.72
List of biological sample collection of rodents
List of Organ and Tissue Matrix for histopathology
Whole blood
Plasma
Serum
White blood cell
Erythrocytes
PBMC
Ocular tissues*
Brain tissue* *
Bone marrow
Organs
Intestinal fluid
Gastric fluid
Bronchoalveolar lavage fluid
Joint lavage fluid
Nasal mucosa
Cerebrospinal fluid
Cerebrospinal fluid (rat multiple consecutive)
Lymph (surgical)
Bile (surgical)
Urine (metabolic cage)
Feces (metabolic cage)
Intestinal perfusion (rat, surgical)
Liver perfusion (rat, surgical)
The samples of ocular tissues include but are not limited to conjunctiva, cornea, iris, lens, ciliary body, retina, choroid, sclera, optic nerve, aqueous humor, vitreous body, and tear.
Samples of various brain tissues include but are not limited to the caudate nucleus, cerebellum, cerebral cortex, white matter of the cerebrum, cingulate gyrus, cingulate sulcus, corpus callosum, external capsule, internal capsule, globus pallidus, hippocampus, hypothalamus, pituitary gland, midbrain, medulla oblongata, optic nerve, optic chiasm, pons, shell, and spinal cord.
Experience
-
18+
Years of experience
-
280+/Year
Rodent IND PK studies
-
10,000+/Year
Rodent discovery PK studies
Facilities
There are 3 facilities for rodent PK studies, which are located in Shanghai, Suzhou, and Nanjing respectively. All animal facilities are barrier systems and are accredited by AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) International. In addition, the Shanghai and Suzhou animal facilities also have the US OLAW Animal Welfare Assurance. The main SPF animals in the facility include rats, mice, guinea pigs, and hamsters, and can support the PK study throughput of 2,200 animals/week.
-


AAALAC Certificate
-


Surgery Room
-


Automated Blood Sampler (ABS)
-


Pulse Vacuum Steam Sterilizer
-


Corridor
-


Cage and Rack washer
-


In vivo imaging system (IVIS)
Learn more about our facilities
Including mouse,rat,hamster,monkey,dog,pig,rabbit,etc
FAQs
-
What is a Rodent PK study?
Rodent pharmacokinetic (PK) studies evaluate and elucidate the mechanism of absorption, distribution, metabolism, and excretion of test articles in rodents, such as mice, rats, etc. These studies are crucial for determining appropriate drug exposure, dose requirements, and other PK parameters to facilitate efficacious and safe studies in the future.
-
What types of rodents are commonly used in PK studies?
In rodent PK studies, the most commonly used animals are mice and rats, followed by hamsters and guinea pigs.
-
What are your capabilities in rodents for neuro PK?
We have a lot of experience with neuro PK studies in rodents, and we can perform intracerebroventricular administration and we can collect cerebrospinal fluid in rats and mice and different brain tissues in rodents as well.
-
What are your advantages in respiratory drug delivery for rodents?
We have rich experience in rodent respiratory drug administration, such as intranasal administration, intratracheal administration, inhalation administration, etc. We have also provided a lot of high-quality study services to customers.
Related Resources




-


Research on the Lymphatic Transport: Enhancing Drug Delivery and Absorption via In Vivo Models
ArticlesFeb 16, 2026Learn More -


Optimization and Assessment of Intrathecal (IT) Administration to Improve Drug Delivery to the CNS in Rats
PostersOct 31, 2025Learn More -


In Vivo Imaging for Small Animals: Deciphering the Spatiotemporal Trajectory of Drugs
ArticlesOct 24, 2025Learn More -


Human Pharmacokinetics Prediction for Trastuzumab Using Scaling and Two-Compartment Modeling with B-hFcRn Mice PK Data
PostersOct 11, 2025Learn More -


What Is Brain Microdialysis and Its Application in PK Studies for CNS Drugs
ArticlesSep 26, 2025Learn More -


Transforming Blood Collection with Automated Blood Sampler (ABS) in Rats
BlogsSep 19, 2025Learn More -


Factsheet-Intrathecal Administration in Rats and Monkeys
BrochuresAug 08, 2025Learn More -


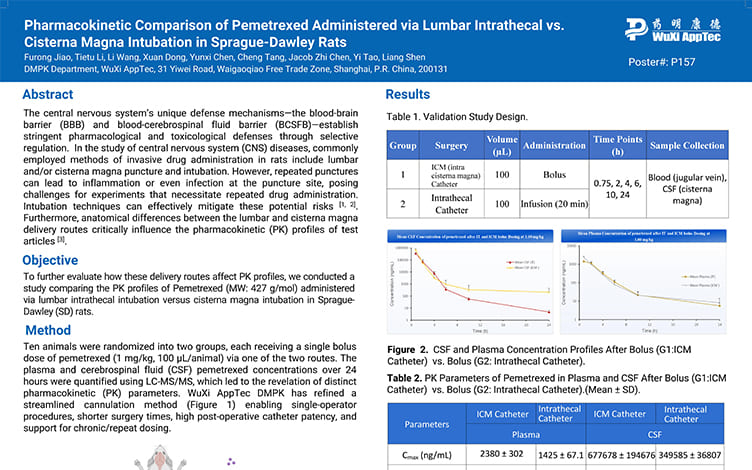
Pharmacokinetic Comparison of Pemetrexed Administered via Lumbar Intrathecal vs.Cisterna Magna Intubation in Sprague-Dawley Rats
PostersJun 12, 2025Learn More -


Effective Intrathecal Injection/Catheterization in Rats for CNS Drugs: Challenges and Strategies
ArticlesJun 12, 2025Learn More -


Small Animal In Vivo Imaging Services
BrochuresMay 28, 2025Learn More -


Effects of Anesthesia and Different Lymphatic Fluid Collection Methods on the Lymphatic Transport of Orally Administered Drugs: A Comparative Study in Rats
PostersOct 30, 2024Learn More -


In Vivo PK | What Are We Striving For?
VideosOct 17, 2024Learn More -


Permeability and Intestinal Absorption Study for Orally Administered Drugs: Preclinical Research Methods and Strategies
ArticlesSep 30, 2024Learn More -


Development and Implementation of an Animal Whole Life Cycle Management System
PostersSep 06, 2024Learn More -


Comparative Evaluation of Blood Collection Methods for Pharmacokinetic Studies in Mice: Serial and Staggered Sampling, and Terminal Blood Sampling
PostersAug 29, 2024Learn More -


Rodent PK Study Part Ⅲ List of Organ and Tissue Matrix for histopathology
BrochuresAug 21, 2024Learn More -


Rodent PK Study Part Ⅱ Surgical Models
BrochuresAug 21, 2024Learn More -


Rodent PK Study Part Ⅰ Route of Administration
BrochuresAug 21, 2024Learn More -


Peptide-Radionuclide Conjugates: Interpretation of FDA Approved Drugs and DMPK Strategies
ArticlesJun 06, 2024Learn More -


Unleashing the Potential of Inhaled Drugs: Challenges and Strategies for Preclinical In Vivo PK Studies
BlogsFeb 27, 2024Learn More -


Inhaled Medications: Challenges and Strategies for Preclinical In Vivo PK Studies
ArticlesJan 12, 2024Learn More -


Why Use Liquid Chromatography-Mass Spectrometry (LC-MS) for Quantitative Pharmacokinetic Analysis of Oligonucleotides
BlogsNov 09, 2023Learn More -


Application of Liquid Chromatography-Mass Spectrometry (LC-MS) in Oligonucleotides Quantitative Analysis
ArticlesOct 19, 2023Learn More -


In Vivo Pharmacokinetic Service
BrochuresJun 21, 2023Learn More
References
- 1.
Guideline, O. E. C. D. 428-Guideline for the Testing of Chemicals-Skin Absorption: in vitro Method." Organization for Economic Cooperation and Development, Paris (2004)
- 2.
Oh, Luke, et al. In Vitro Skin Permeation Methodology for Over-The-Counter Topical Dermatologic Products." Therapeutic Innovation & regulatory science 54.3 (2020): 693-700
- 3.
Kim, J.S., Mitchell, S., Kijek, P., Tsume, Y., Hilfinger, J. and Amidon, G.L., 2006. The suitability of an in-situ perfusion model for permeability determinations: utility for BCS class I biowaiver requests. Molecular Pharmaceutics, 3 (6), pp.686-694
- 4.
Chiou, W.L. and Barve, A., 1998. Linear correlation of the fraction of oral dose absorbed of 64 drugs between humans and rats. Pharmaceutical Research, 15 (11), p.1792
- 5.
Cao, X., Gibbs, S.T., Fang, L., Miller, H.A., Landowski, C.P., Shin, H.C., Lennernas, H., Zhong, Y., Amidon, G.L., Lawrence, X.Y. and Sun, D., 2006. Why is it challenging to predict intestinal drug absorption and oral bioavailability in humans using the rat model. Pharmaceutical Research, 23 (8), pp.1675-1686
- 6.
Salphati, L., Childers, K., Pan, L., Tsutsui, K. and Takahashi, L., 2001. Evaluation of a single-pass intestinal perfusion method in rats for the prediction of absorption in man. Journal of pharmacy and pharmacology, 53 (7), pp.1007-1013
- 7.
Dahlgren, D., Roos, C., Sjögren, E. and Lennernäs, H., 2015. Direct in vivo human intestinal permeability (Peff) determination with different clinical perfusion and intubation methods. Journal of pharmaceutical sciences, 104 (9), pp.2702-2726
Stay Connected
Keep up with the latest news and insights.