-
Overview
-
Services
-
Featured Strengths
-
FAQs
-
Related Resources
-
Related Services
Overview
The WuXi AppTec DMPK is committed to providing a comprehensive R&D platform for in vivo PK experiments assisting new drug R&D projects with the most effective in vivo research methods. The protocol is designed specifically to fulfill clients' needs and their experiment purposes, including formulation screening, dose and sampling time point setting, administration route, and sample matrix selection. In formulation screening studies, the compound stability and solubility in its solvent are measured based on its physicochemical properties before in vivo study starts. DMPK providing accurate and efficient solutions to cope with all the challenges that occur in the new drug development.
Learn More


Services
-


Learn More
Rodent PK study
Species: Mouse, Rat, Hamster, Guinea pig
Route of Administration: IV bolus, Oral administration, 7-day continuous intravenous infusion,Osmotic Pumps Infusion, Capsule administration, Intrathecal injection, Intratracheal administration
Surgery Model: Jugular Vein Cannulation, Carotid Artery Cannulation,Bile Duct Cannulation, Portal Vein Cannulation, Lymph Duct Cannulation
-


Learn More
Large Animal (Non-rodent) PK study
Species: Monkey, Dog, Pig, Rabbit, Ferret
Route of Administration: Intravenous bolus, Intravenous Infusion, 24 hours continuous intravenous infusion
Surgery Model: Cisterna Magna Cannulation, Bile Duct Cannulation, Portal Vein Cannulation, Lymph Duct Cannulation, Intestinal Cannulation
-


Learn More
Preclinical Formulation Screening
Physicochemical Properties Characterization: pKa, Log D/P, Solubility, Stability
Preclinical Formulation Screening: Clear solution, Suspension, CDs, SEDDS
Preclinical Formulation Optimization: Micronization, Solid dispersion, Capsules, Nanosuspension
-


Learn More
Clinicopathological Testing Services
Service items: Hematology, biochemistry, coagulation, urinalysis. Biochemical tests can be conducted for panel function including blood lipids, liver function, kidney function, electrolytes, urinary kidney function, and special proteins.
Animal species: Mouse, Rat, Dog, Monkey, Pig
Featured Strengths
High Standards of Animal Welfare
The animal facilities meet with China, US, and EU standards
Good animal care and use program with oversight of animal welfare
Learn More


-


Abundant Animal Resources
More than 10,000 rodents and more than 3000 non-rodents in stock, no lead time
-


Extensive Surgery Experience
Equipped with state-of-the-art animal surgery rooms, 15+ years of animal surgery experience
-


Automated Facilities
Chips and intelligent cage cards are utilized to manage large animals, enhancing efficiency and quality management
-


Preclinical Vehicle Screening Service
Experienced in preclinical vehicle screening for different types of test articles with safe and effective vehicles
FAQs
-
What is a pharmacokinetic study?
Drug metabolism and pharmacokinetic studies primarily characterize the absorption, distribution, metabolism, and excretion (ADME) properties of compounds, playing a crucial role in reducing the failure rate of drug development. Clarifying and optimizing the pharmacokinetic properties of drugs can help select drugs with better target efficacy and safety, reduce the risk of drug interactions, and provide a basis for clinical dosing and frequency.
-
What is the difference between in vivo and in vitro pharmacokinetics?
In vivo and in vitro pharmacokinetics refer to different methods of studying how a drug behaves in a biological system. Both In vivo and in vitro studies are important and complementary in the field of pharmacokinetics.
In vivo pharmacokinetics involves studying the drug in a living organism. This could be in humans, animals, or even plants. The drug is administered and then various measurements are taken to understand how the drug is absorbed, distributed, metabolized, and excreted. This provides a comprehensive view of the drug’s behavior in a complex biological system, including the effects of various physiological processes. In contrast, in vitro pharmacokinetics involves studying the drug outside of a living organism, often in a controlled laboratory environment such as a petri dish or test tube. These studies typically involve using isolated cells, tissues, or enzymes to understand specific aspects of the drug’s behavior, such as its metabolism or interaction with certain proteins.
In vitro studies can provide more controlled and specific information, but they lack the complexity of a whole organism. Conversely, in vivo studies provide a more holistic view, but can be more complex and difficult to interpret due to the multitude of factors at play.
Related Resources




-


Research on the Lymphatic Transport: Enhancing Drug Delivery and Absorption via In Vivo Models
ArticlesFeb 16, 2026Learn More -


Liposome Drug Delivery: Classification, Composition, and Formulation Considerations
ArticlesJan 16, 2026Learn More -


The Role of Lymphatic Transport on the Systemic Bioavailability of the Bcl-2 Protein Family Inhibitors Navitoclax (ABT-263) and ABT-199
PublicationsJan 16, 2026Learn More -


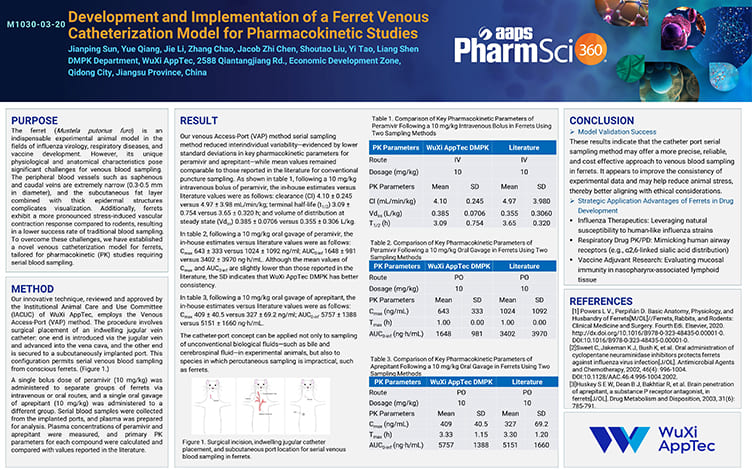
Development and Implementation of a Ferret Venous Catheterization Model for Pharmacokinetic Studies
PostersNov 28, 2025Learn More -


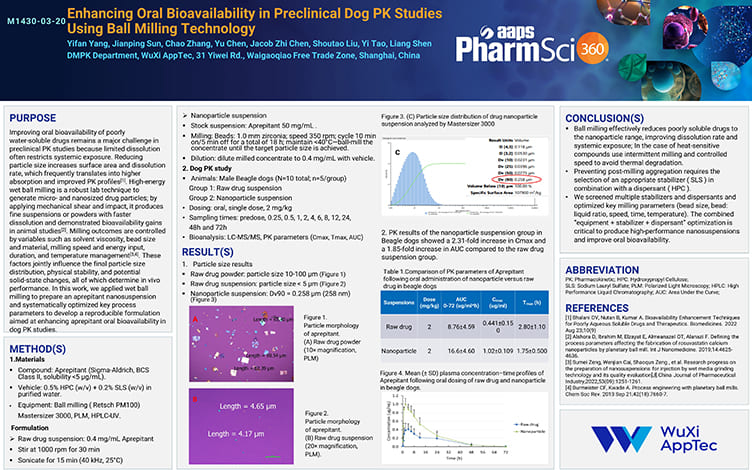
Enhancing Oral Bioavailability in Preclinical Dog PK Studies Using Ball Milling Technology
PostersNov 28, 2025Learn More -


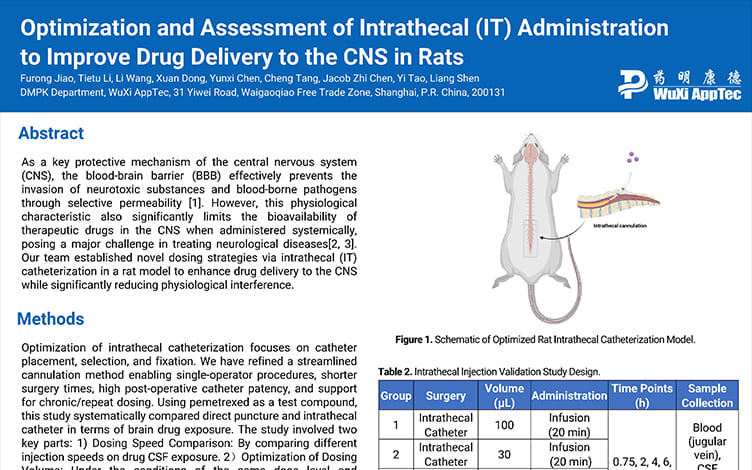
Optimization and Assessment of Intrathecal (IT) Administration to Improve Drug Delivery to the CNS in Rats
PostersOct 31, 2025Learn More -


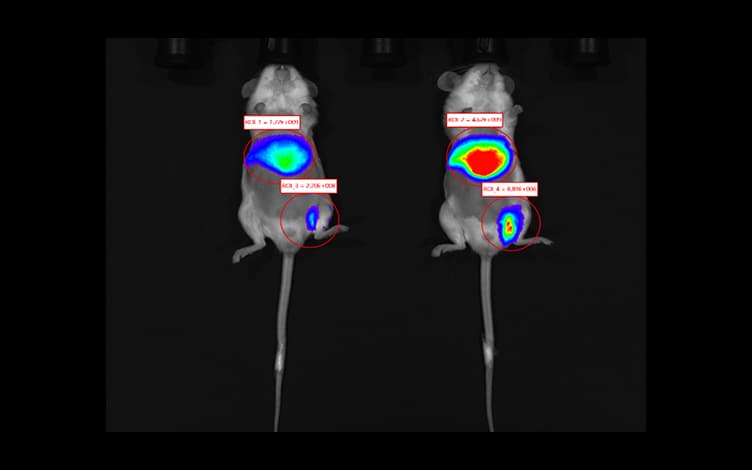
In Vivo Imaging for Small Animals: Deciphering the Spatiotemporal Trajectory of Drugs
ArticlesOct 24, 2025Learn More -


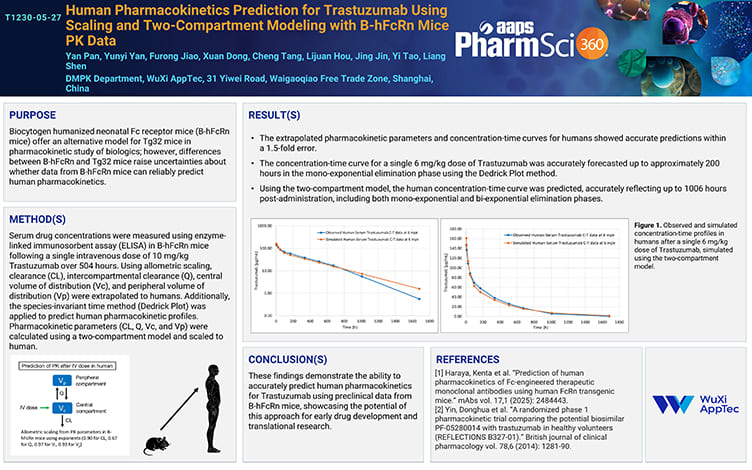
Human Pharmacokinetics Prediction for Trastuzumab Using Scaling and Two-Compartment Modeling with B-hFcRn Mice PK Data
PostersOct 11, 2025Learn More -


What Is Brain Microdialysis and Its Application in PK Studies for CNS Drugs
ArticlesSep 26, 2025Learn More -


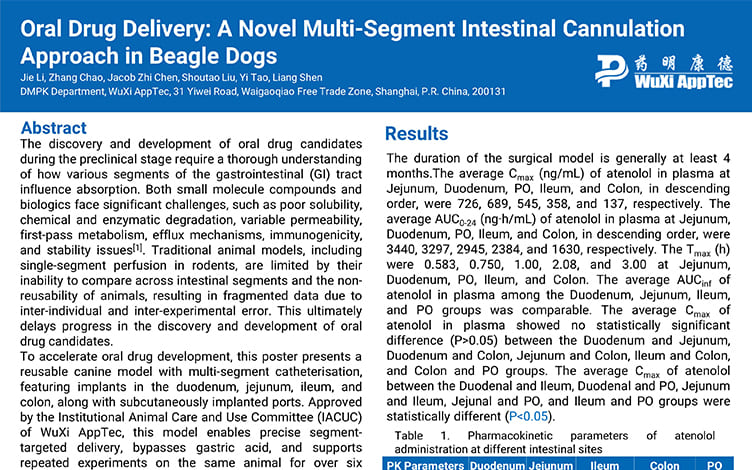
Oral Drug Delivery: A Novel Multi-Segment Intestinal Cannulation Approach in Beagle Dogs
PostersSep 19, 2025Learn More -


Transforming Blood Collection with Automated Blood Sampler (ABS) in Rats
BlogsSep 19, 2025Learn More -


Particle Size Analysis and Reduction Techniques to Enhance Drug Bioavailability in Preclinical Studies
ArticlesSep 05, 2025Learn More -


Evaluation of the Safety and Cross-Species Tolerability of Solutol HS 15 via Intravenous Administration in Various Animals
PostersAug 28, 2025Learn More -


Evaluating Oral Peptides' Gastrointestinal Absorption: Methods and Applications
ArticlesAug 28, 2025Learn More -


Discovery of Orally Bioavailable Ligand Efficient Quinazolindiones as Potent and Selective Tankyrases Inhibitors
PublicationsAug 28, 2025Learn More -


Factsheet-Intrathecal Administration in Rats and Monkeys
BrochuresAug 08, 2025Learn More -


Minimizing Hemolysis in In Vivo Pharmacokinetic Studies: Causes, Assessment, and Optimal Vehicle and Additive Selection
ArticlesAug 08, 2025Learn More -


More Accurate Prediction of Human Pharmacokinetic Parameters for Small Molecule Compounds: Methods, Models, and Applications
ArticlesAug 01, 2025Learn More -


New Book Release: Drug Metabolism and Pharmacokinetics: Frontiers, Strategies, and Applications | WuXi AppTec DMPK
VideosJul 17, 2025Learn More -


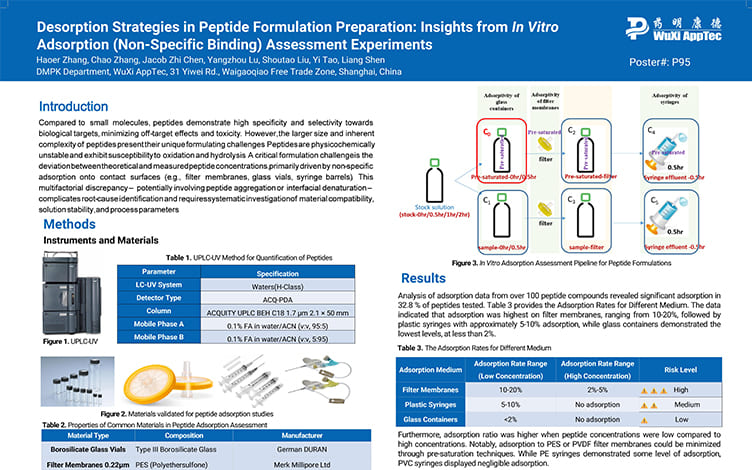
Desorption Strategies in Peptide Formulation Preparation: Insights from In Vitro Adsorption (Non-Specific Binding) Assessment Experiments
PostersJul 17, 2025Learn More -


Decoding DMPK Frontiers for Novel Therapeutics: WuXi AppTec DMPK’s New Book Release
BlogsJul 10, 2025Learn More -


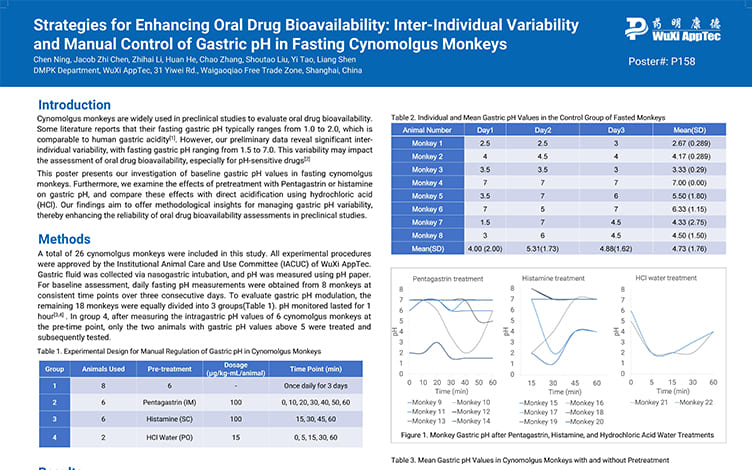
Strategies for Enhancing Oral Drug Bioavailability: Inter-Individual Variability and Manual Control of Gastric pH in Fasting Cynomolgus Monkeys
PostersJun 20, 2025Learn More -


Drug Metabolism and Pharmacokinetics: Frontiers, Strategies, and Applications
PublicationsJun 13, 2025Learn More -


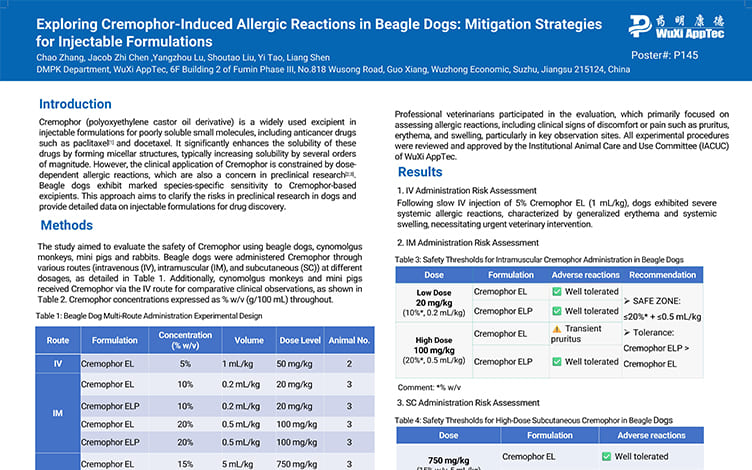
Exploring Cremophor-Induced Allergic Reactions in Beagle Dogs: Mitigation Strategies for Injectable Formulations
PostersJun 12, 2025Learn More -


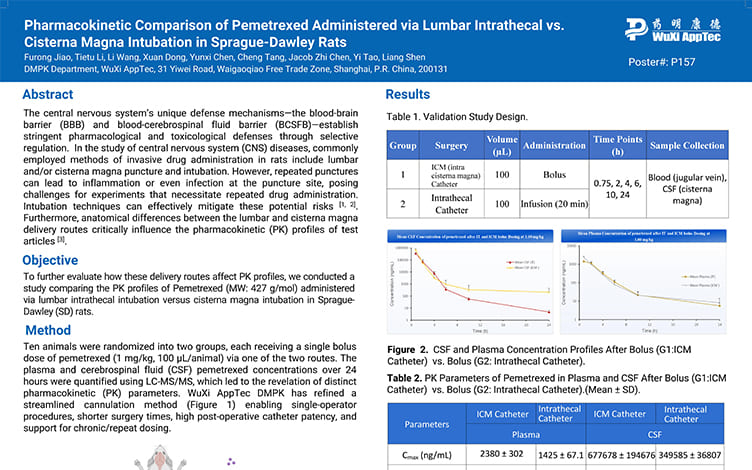
Pharmacokinetic Comparison of Pemetrexed Administered via Lumbar Intrathecal vs.Cisterna Magna Intubation in Sprague-Dawley Rats
PostersJun 12, 2025Learn More -


Effective Intrathecal Injection/Catheterization in Rats for CNS Drugs: Challenges and Strategies
ArticlesJun 12, 2025Learn More -


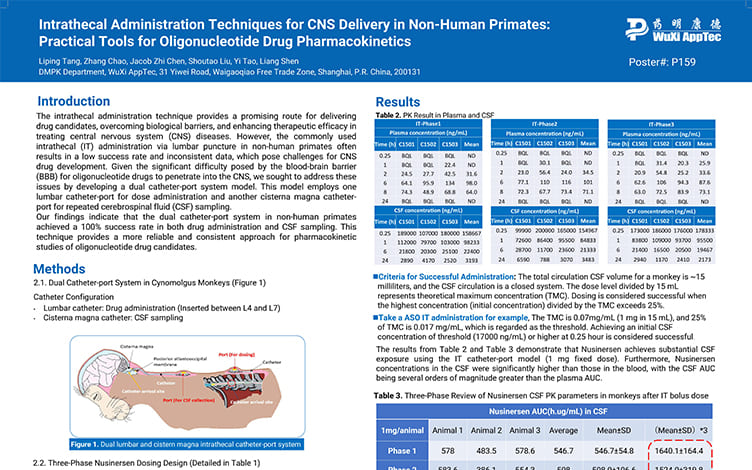
Intrathecal Administration Techniques for CNS Delivery in Non-Human Primates:Practical Tools for Oligonucleotide Drug Pharmacokinetics
PostersJun 05, 2025Learn More -


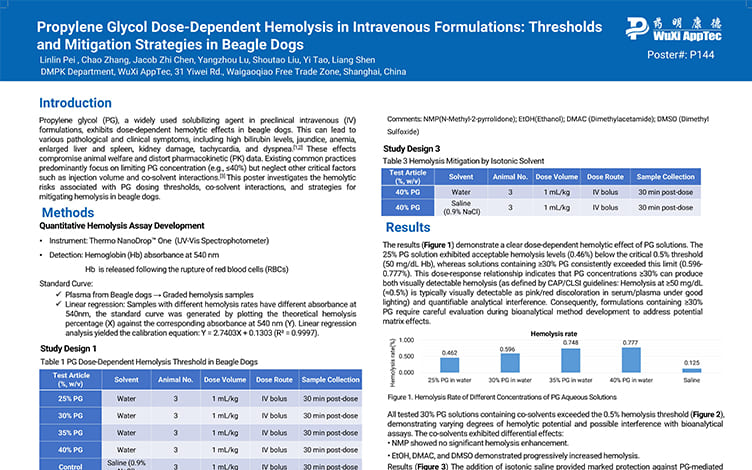
Propylene Glycol Dose-Dependent Hemolysis in Intravenous Formulations: Thresholds and Mitigation Strategies in Beagle Dogs
PostersJun 05, 2025Learn More -


Small Animal In Vivo Imaging Services
BrochuresMay 28, 2025Learn More -


Intrathecal Administration: Breaking Barriers for Central Nervous System Drugs
VideosMay 28, 2025Learn More -


High-Performance, Professional, Innovative: WuXi AppTec DMPK Accelerates Breakthroughs
VideosMay 22, 2025Learn More -


FAQs on Oligonucleotide DMPK Studies Stability, Distribution, Administration, and Bioanalysis
BlogsApr 03, 2025Learn More -


The Impact of Gastrointestinal pH on Oral Drug Absorption
ArticlesMar 28, 2025Learn More -


Advancements in Pet Drugs and Pharmacokinetic Insights from FDA-Approved Products
ArticlesFeb 06, 2025Learn More -


WuXi AppTec DMPK: Your Trustworthy Partner
VideosDec 27, 2024Learn More -


Application and Best Practices of Liver Biopsy in Non-Rodents
BlogsNov 28, 2024Learn More -


4 Best Practices for Intrathecal Administration in CNS Drug Development
BlogsNov 12, 2024Learn More -


Effects of Anesthesia and Different Lymphatic Fluid Collection Methods on the Lymphatic Transport of Orally Administered Drugs: A Comparative Study in Rats
PostersOct 30, 2024Learn More -


In Vivo PK | What Are We Striving For?
VideosOct 17, 2024Learn More -


Permeability and Intestinal Absorption Study for Orally Administered Drugs: Preclinical Research Methods and Strategies
ArticlesSep 30, 2024Learn More -


Evaluation of Different Tissue Processing Methods in Bama Pig Skin as an Animal Model for Topical Delivery Systems
PostersSep 19, 2024Learn More -


Development and Implementation of an Animal Whole Life Cycle Management System
PostersSep 06, 2024Learn More -


Comparative Evaluation of Blood Collection Methods for Pharmacokinetic Studies in Mice: Serial and Staggered Sampling, and Terminal Blood Sampling
PostersAug 29, 2024Learn More -


Application of Innovative Intelligent Electronic Cage Cards in Large-Scale Animal Facilities
PostersAug 25, 2024Learn More -


Rodent PK Study Part Ⅲ List of Organ and Tissue Matrix for histopathology
BrochuresAug 21, 2024Learn More -


Rodent PK Study Part Ⅱ Surgical Models
BrochuresAug 21, 2024Learn More -


Rodent PK Study Part Ⅰ Route of Administration
BrochuresAug 21, 2024Learn More -


Large Animal (Non-Rodent) PK Study Part Ⅲ List of Organ and Tissue Collection
BrochuresAug 21, 2024Learn More -


Large Animal (Non-Rodent) PK Study Part Ⅱ Surgical Models
BrochuresAug 21, 2024Learn More -


Large Animal (Non-Rodent) PK Study Part Ⅰ Route of Administration
BrochuresAug 21, 2024Learn More
Stay Connected
Keep up with the latest news and insights.