-
Overview
-
Study Models and Platforms
-
Experience
-
Study Strategies and Assays
-
Case Studies
-
FAQs
-
Related Resources
-
Related Services
Overview
WuXi AppTec DMPK has established an integrated platform for ADCs, which includes payload release studies in a variety of in vitro systems, ADME and PK studies for both payload and ADC, and comprehensive bioanalysis platform for the detection of total antibody, conjugated antibody, free payload, conjugated payload, drug-antibody ratio (DAR), and so on, as well as metabolite identification studies. Based on the research platform, WuXi AppTec DMPK has supported more than 200 ADCs for screening and more than 20 ADCs for IND filing. Throughout this process, we have accumulated extensive experience and can propose a specific drug metabolism and pharmacokinetics (DMPK) strategy for each ADC project, which can effectively shorten the ADC development cycle.
Learn More

Study Models and Platforms
-
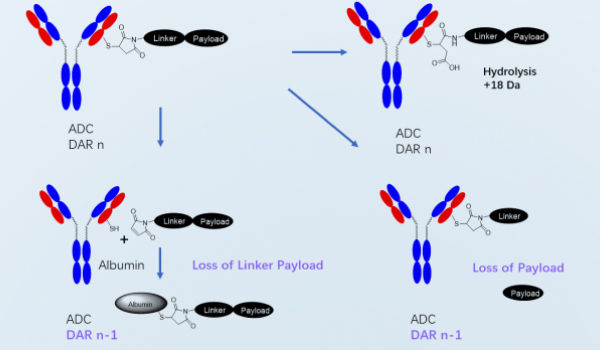
In vitro stability research platform in blood matrix
The stability of ADCs in the blood matrix can help assess the stability of ADCs in the circulatory system, assess the off-target risk and species differences. WuXi AppTec DMPK has established various in vitro systems for evaluating the stability of ADCs, including whole blood, plasma, and serum, and also has the capability to detect a variety of analytes, such as free payload, ADC, total antibody, DAR value and identification of payload-related metabolites, etc. These capabilities enable us to provide comprehensive evaluations of the in vitro stability of ADCs, thereby assisting in the early evaluation of ADC candidates.
-
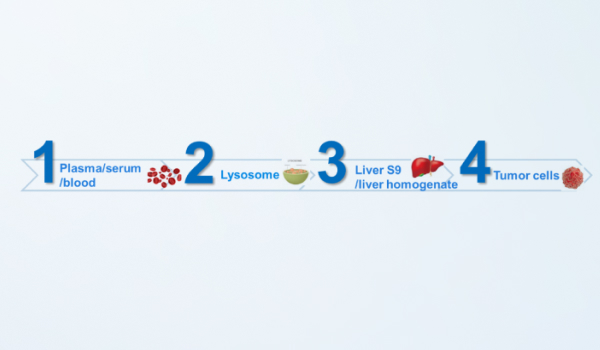
Payload release study platform
In vitro assessment of payload release of ADC is helpful to understand the cleavage mode of ADC and identify the payload-related species after cleavage, which provides data support for the detection or evaluation of pharmacokinetic/toxokinetic studies. WuXi AppTec DMPK has established various systems to evaluate payload release in lysosome, liver S9, liver homogenate or tumor cells. Detection of known free small molecules, as well as metabolite identification studies on payload-related species can be performed to fully evaluate payload release of ADC.
-
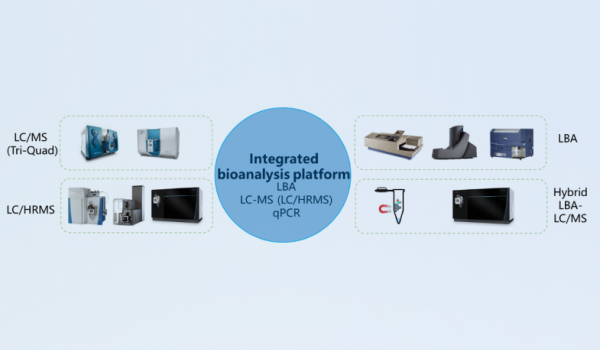
Integrated bioanalysis platform
Not only large molecules (total antibody and conjugated antibody) but also small molecules (free payload and related species) are quantified for ADC project. Quantitative bioanalysis of total antibody, conjugated antibodies, payload, and relevant metabolites is run throughout all stages of ADC project development. In order to correctly evaluate the ADME characteristics of ADCs, comprehensive bioanalytical methods are required. The ADC integrated bioanalysis platform, established by WuXi AppTec DMPK, provides various analysis services, including ligand binding analysis (LBA), liquid chromatography mass spectrometry, liquid chromatography high-resolution mass spectrometry, and Hybrid LBA-LC/MS. The integrated bioanalysis platform enables the detection of total antibody, conjugated antibody, immunogenicity, payload and payload-related species, DAR value, etc. and facilitates metabolite identification and biotransformation analysis.
-
Radiolabeled ADME studies platform
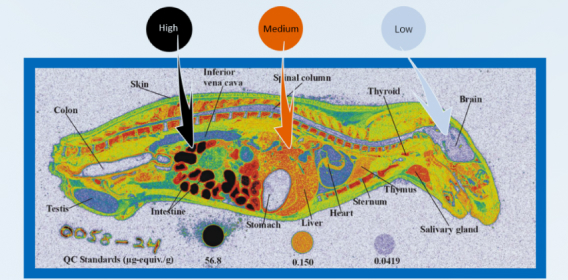
If new paylaod-related species is genearated as main payload-release product after in vivo cleavage of ADC, it is necessary to evaluate the plasma protein binding, tissue distribution, metabolism, excretion/mass balance, drug metabolizing enzymes and transporter effects. In vivo ADME of the payload is normally carried out by using radioisotope labeling trace technology, including specific tissue distribution studies to elucidate the targeting characteristics.
WuXi AppTec DMPK has established the platform of 14C/3H labeled ADC synthesis, mass balance, tissue distribution (including tumor-bearing mice), and metabolite identification studies in small and large animals, to comprehensively evaluate the ADME characteristics of ADC in animals.
Experience
-
10+
Years’ experience in ADC drug development
-
20+
ADCs for IND filing
-
60+
Global clients of ADC
Study Strategies and Assays
-
The development of ADC involves quite a lot of pharmacokinetic studies, but the pharmacokinetic studies for ADC should be selective. Designing customized strategies based on the characteristics of the specific ADC will help accelerate the development of the ADC.

EXPLORATORY Lead Molecule Optimization | PRE-CLINIC IND Enabling and IND Filing | |
ADME | Study on the payload release from ADC in vitro Stability of ADC in plasma/serum/blood Metabolic stability of payload | Metabolite identification of payload in liver microsomes and hepatocytes Plasma protein binding of payload ADME studies of radiolabeled payload in animals Biodistribution studies of radiolabeled ADC in tumor-bearing animals |
In Vitro DDI | In vitro efflux transporters substrate evaluation of payload In vitro permeability of payload | In vitro CYP450 inhibition and induction of payload CYP450 enzyme phenotyping of payload In vitro transporter inhibition of payload |
PK | PK studies of ADC and payload in rodents PK studies of ADC in pharmacological model | PK studies of ADC or payload in non-rodents PK/PD studies of ADC |
Case Studies
-
In vitro stability of ADC
-
In vivo PK study of ADC
-
-

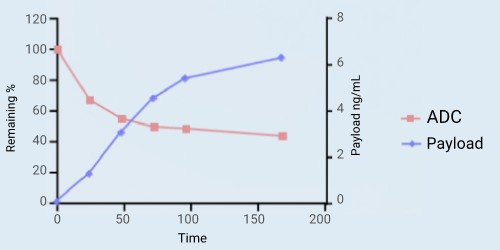
Background:
ADCs are expected to remain stable in the circulatory system to avoid adverse reactions caused by premature release of payloads. Blood matrix is a simple and direct method to evaluate the stability of ADC in vitro. Most of the ADCs on the market have been tested for their stability in vitro using plasma or serum.
Experimental design:
Commercial ADC A at the final concentration of 100 μg/mL was incubated in human plasma for 7 days. Bioanalysis of payload was carried out in a Waters Aquity UPLC liquid phase system coupled with the SCIEX 6500+ mass spectrometry and the chromatography was performed on ACQUITY UPLC Protein BEH C4 300A 1.7 μm 2.1 * 50 mm Column. Bioanalysis of ADC was performed by using a ligand binding method. The trapping reagent was Anti-payload monoclonal antibody (clone 3F3) and the detection reagent was Goat Anti-Human IgG HRP.
Results:
Within 7 days of incubation, payload continued to release slowly. However, the concentration of ADC decreased more rapidly, and about 40% of ADC was remained after 7 days of incubation.
Learn More
-
-
-

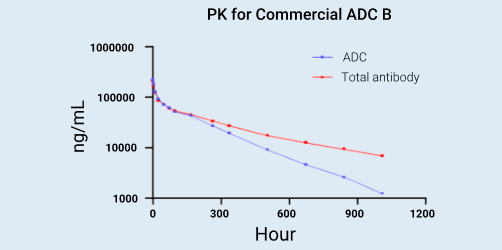
Background:
ADCs have complex pharmacokinetic profiles, and the basis approach for bioanalysis is to measure the concentration of ADCs and Total Antibody (TAb) as well as deconjugated warhead in the circulation. Conjugated antibody means an antibody that is coupled to at least one payload, and total antibody is the sum of conjugated antibody and unconjugated antibody. By definition, concentration of total antibody is always greater than that of conjugated antibody.
Experimental design:
Commercial ADC B was intravemously administrated to SD rats at the dosage of 10 mg/kg, and 15 serum samples per animal were collected from 0.0833h to 1008h post dosing. Both ADC and total antibody were analyzed by using ligand binding method. The capturing reagent of total antibody was Human Target Protein, and the detecting reagent was Goat Anti-Human IgG HRP. The conjugated antibody was captured by Anti-Payload Mab.G2E6 and detected by Goat Anti-Human IgG HRP.
Results:
Comparison of conjugated antibody PK and total antibody PK after ADC administration provides information regarding the stability of ADC. The degree of divergence of the curves is indicative of the rate of complete payload loss from the ADC. As showed in the picture, conjugated antibody concentration and total antibody concentrations are similar at the initial phase of the PK profile. Over time, conjugated antibody showed a quicker clearance than total antibody, it means that more antibody with complete payload loss was existed.
Learn More
-
FAQs
-
What is an antibody drug conjugate?
Antibody-drug conjugates (ADCs) are promising cancer treatments consisted of cytotoxic drugs (payloads) covalently bind to monoclonal antibodies (mAbs) through linkers. ADCs enable the selective delivery of highly cytotoxic payloads to tumor cells thar express particular antigen.
-
What are the advantages of antibody drug conjugates?
ADC drugs are superior to either antibody drugs or chemotherapy alone by overcoming their limitations while preserving the merits from both, which can specifically deliver the cytotoxic payload to the target cells and significantly expand the therapeutic window of a chemotherapeutic by minimizing the systemic exposure and associated toxicity of the chemotherapeutic agent.
-
What diseases are treated with ADCs?
ADC drugs were developed to treat a wide range of hematologic and solid tumors, which includes acute myeloblastic and lymphoblastic leukemias, various types of lymphoma, breast, gastric or gastroesophageal junction, lung, urothelial, cervical, and ovarian cancers.
Related Resources




-


A Review on ADC Linkers: Cleavable and Non-Cleavable Linker Design and Stability Evaluation
ArticlesJan 30, 2026Learn More -


What Are Antibody–Peptide Conjugates (APCs) Drugs and Their Pharmacokinetic Properties
ArticlesNov 21, 2025Learn More -


Integrated Bioanalytical Platform and DMPK Strategies for Antibody-Drug Conjugates
WebinarsNov 13, 2025Learn More -


In Vitro Platform for Assessing ADC Stability and Payload Release
PostersOct 31, 2025Learn More -


Rapid Screening and Characterization of Payload-Related Catabolites Derived from ADCs in In Vitro Models by UPLC-UV-HRMS
PostersOct 22, 2025Learn More -


Bioanalytical Strategy for Antibody-Drug Conjugates (ADCs) Based on Integrated Mass Spectrometry Bioanalysis Platform
PostersSep 11, 2025Learn More -


Advancing Novel Therapeutics with Innovative DMPK Strategies in Metabolism and Bioanalysis
WebinarsAug 22, 2025Learn More -


Bioanalytical Strategy for Payload-Related Species of Antibody Drug Conjugates (ADCs) with LC-MS
PostersAug 01, 2025Learn More -


Antibody-Drug Conjugate (ADC) R&D Innovations and In Vitro ADME Research Considerations
ArticlesJul 24, 2025Learn More -


8 Commonly Used ADC Payloads and Their ADME and DDI Properties
ArticlesMay 22, 2025Learn More -


Detection and Characterization of In Vitro Payload-Containing Catabolites of Noncleavable Antibody-Drug Conjugates by High-Resolution Mass Spectrometry and Multiple Data Mining Tools
PublicationsApr 28, 2025Learn More -


Immunogenicity Testing: Definition, Guidelines, and Anti-Drug Antibody Detection
ArticlesApr 25, 2025Learn More -


Optimization of the LLOQ for Exatecan, a Widely-Used Antibody Drug Conjugate (ADC) Payload
PostersMar 12, 2025Learn More -


Bioanalytical Strategy for Quantitative Conjugated Payloads of Antibody-Drug Conjugates (ADCs) with Cleavable Linker
PostersMar 12, 2025Learn More -


Streamlining the Bioanalysis of Antibody-Drug Conjugates (ADCs): Simultaneous Determination of Total Antibodies and Conjugated Payloads
ArticlesMar 04, 2025Learn More -


Antibody-Drug Conjugate (ADC) Preclinical PK/PD Study Strategies and Practices
ArticlesFeb 19, 2025Learn More -


Development of a Rapid, High Throughput Quantitative Bioanalysis Method for Conjugated Payloads of Protease-Cleavable Antibody-Drug Conjugate (ADC) in Rat Serum Using Automated Immuno-Capture Based UPLC-MS/MS
PostersFeb 06, 2025Learn More -


Comprehensive Mass Spectrometry Based Bioanalysis Strategy for Antibody-Drug Conjugate Therapeutics
PostersJan 30, 2025Learn More -


Methodologies and Strategies for ADC Biotransformation Studies
WebinarsJan 15, 2025Learn More -


Advancing Antibody-Drug Conjugates (ADCs) with Novel Payloads and Their DMPK Considerations
ArticlesDec 10, 2024Learn More -


Spotlight on the DMPK and Bioanalysis Strategy of Antibody Oligonucleotide Conjugates (AOCs)
ArticlesNov 21, 2024Learn More -


In Vitro Metabolic Stability Evaluation of ADCs in Plasma and Whole Blood
PostersOct 17, 2024Learn More -


A Novel Validated LC-MS/MS Approach for Simultaneous Determination of Total Antibody and Conjugated Payload for DS-8201 in SD Rat Serum
PostersAug 08, 2024Learn More -


Webinar Q&A | DMPK Strategies in the Preclinical Development of Antibody-Drug Conjugates
BlogsJun 14, 2024Learn More -


Facilitating ADC Preclinical DMPK Research with an Integrated Bioanalysis Platform
WebinarsMay 24, 2024Learn More -


Webinar Q&A | Facilitating ADC Preclinical DMPK Research with an Integrated Bioanalysis Platform
BlogsMay 24, 2024Learn More -


DMPK Strategies in the Preclinical Development of Antibody-Drug Conjugates
WebinarsMar 25, 2024Learn More -


DAR Distribution Determination for Antibody-drug-conjugates (ADCs) by LC-HRMS in In Vitro and In Vivo Studies
PostersOct 27, 2023Learn More -


Webinar Q&A | The Strategies and Methods for In Vitro ADME Characterization of ADCs
BlogsAug 23, 2023Learn More -


The Importance of Drug Conjugate Linker Design: Effect on the Pharmacokinetic (PK), Drug Efficacy, and Toxicity Profiles
BlogsAug 18, 2023Learn More -


The Strategies and Methods for In-Vitro ADME Characterization of ADCs
WebinarsAug 18, 2023Learn More -


Quantitative Whole-body Autoradiography (QWBA) Enables ADC Tissue Distribution Studies in Tumor-bearing Nude Rodents
BlogsAug 18, 2023Learn More -


Using Radiolabeling Techniques to Improve ADC Pharmacokinetic Studies
BlogsAug 18, 2023Learn More -


A New Method to Improve Identification of the Payload-Containing Catabolites of ADCs
BlogsAug 10, 2023Learn More -


Comprehensive Bioanalysis of ADCs in DMPK Studies
ArticlesAug 10, 2023Learn More -


Unlocking the Mysteries of ADC PK: Strategies for ADC Bioanalysis Based on LC-MS Strategies
BlogsAug 10, 2023Learn More -


Drug Conjugate Linkers and Their Effects on Drug Properties
ArticlesAug 10, 2023Learn More -


Strategies for ADC Payload Release and Metabolism: A Key Step in the Development
BlogsAug 02, 2023Learn More -


Application of Radiolabeling Techniques in ADC PK Studies
ArticlesJul 26, 2023Learn More -


Antibody-Drug Conjugate (ADC) Bioanalysis Strategies Based on LC-MS
ArticlesJul 20, 2023Learn More -


Strategies and Methods for Studying ADC Payload Release and Metabolism
ArticlesJul 13, 2023Learn More -


In Vitro/Vivo Characterization of ADC Drugs by Immunocapture and High-resolution Mass Spectrometry
PostersMay 19, 2023Learn More -


Integrated Bioanalytical Platform Accelerates ADC IND Filing
PostersMay 12, 2023Learn More -


Preclinical Drug Development Testing for Antibody-Drug Conjugate (ADC)
BrochuresMay 04, 2023Learn More
Stay Connected
Keep up with the latest news and insights.