-
Overview
-
Strengths
-
Instrument Platforms
-
FAQs
-
Related Resources
-
Related Services
DMPK BA
Overview
Our team has established a variety of advanced LC-MS platforms to meet customers' analytical needs at different stages. We have a wide range of mass spectrometers, such as multiple generations of Sciex triple quadrupole mass spectrometers, PerkinElmer NexION 2000 ICP-MS, Waters XeVo G2 QToF, Vion QToF, and ThermoFisher Orbitrap Q-Exactive™ Plus, Q-Exactive™ HF, and Eclipse systems. These mass spectrometry systems are coupled with ultrahigh-performance liquid chromatography systems such as Waters UPLC and Shimadzu UHPLC. For the screening stage in vitro sample bioanalysis, high throughput autosamplers such as CTC and ADDA are utilized. Molecular Devices SpectraMax M5/M5e microplate readers, Meso Scale Discovery SECTOR S 600 (which is capable for 384-well format assay) and QuickPlex SQ120, and BD LSRFortessa™ cell analyzer are applied for the LBA analysis of large molecules and cells. In addition, we have multiple automated sample preparation systems, such as the HP D300e instruments, Hamilton Microlab STAR™ and STARLET™ liquid handler workstations, and Night Watcher Full Automation System, to support fast sample preparation.
Learn More


Strengths
-
10 +
Types of bioanalysis platforms
-
200+
Analytical instruments
-
24 h
Fully automated sample preparation
Instrument Platforms
-
-


Waters Vion™ QTof
-


Orbitrap Eclipse™ Tribrid™
-


Sciex Triple Quad™ 7500
-


PE NexION 2000
-
-
-


MESO Sector S 600MM
-


Molecular Devices SpectraMax M5e
-


BD LSRFortessa™ X-20
-
-
-


QIAcube HT
-


QuantStudio™ 7 Flex Real-Time PCR System
-


QIAgility
-
-
-


Apricot Designs Dual Arm
-


High-throughput intelligent delivery system
-
FAQs
Related Resources




-


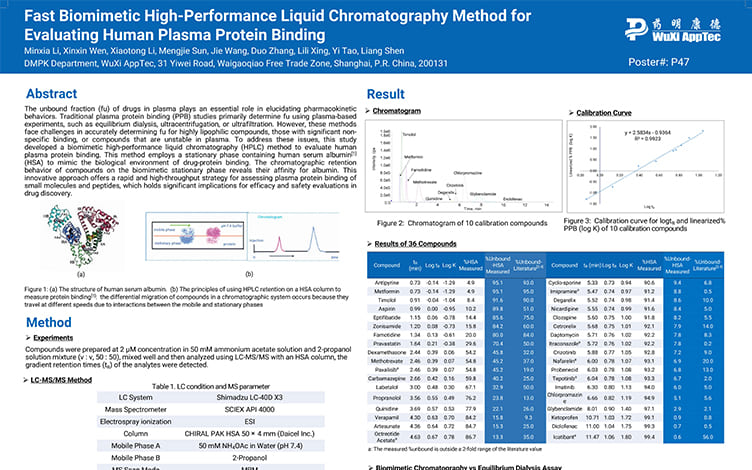
Fast Biomimetic High-Performance Liquid Chromatography Method for Evaluating Human Plasma Protein Binding
PostersSep 30, 2025Learn More -


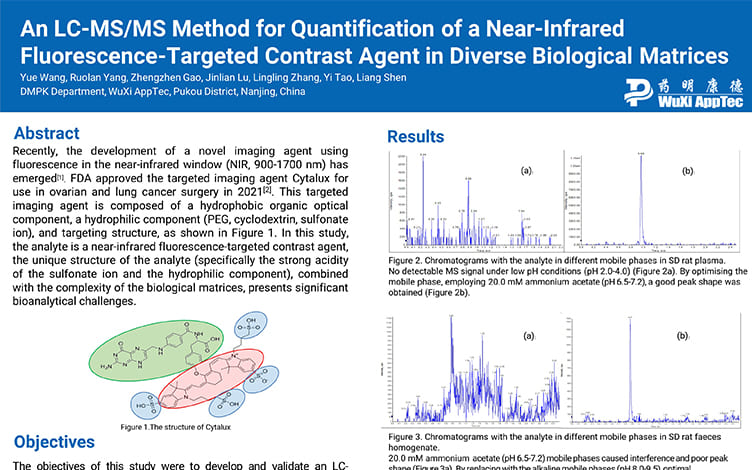
An LC-MS/MS Method for Quantification of a Near-Infrared Fluorescence-Targeted Contrast Agent in Diverse Biological Matrices
PostersAug 25, 2025Learn More -


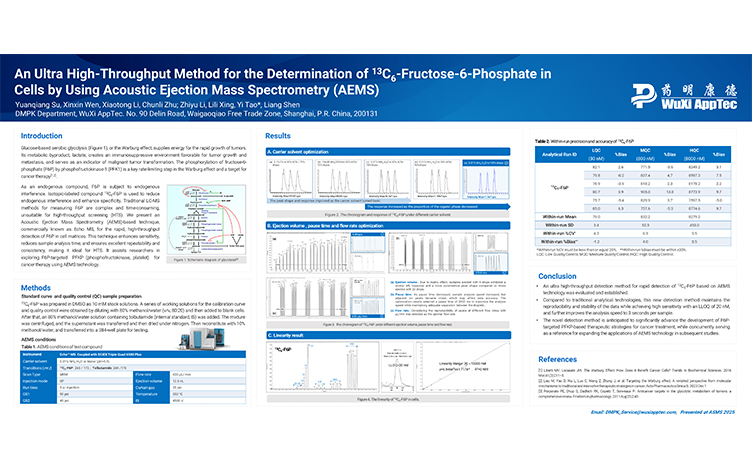
An Ultra High-Throughput Method for 13C6-Fructose-6-Phosphate in Cells Using Acoustic Ejection Mass Spectrometry (AEMS)
PostersJul 04, 2025Learn More -


Construction of a UPLC-UPC2-MS/MS Platform
PostersJun 27, 2025Learn More -


Overview of Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Its Applications in Bioanalysis
ArticlesFeb 26, 2025Learn More
Stay Connected
Keep up with the latest news and insights.