-
Overview
-
Assays
-
Study Strategies
-
Case Study
-
Regulatory Guidance
-
Experience
-
Instruments and Software
-
FAQs
-
Related Resources
-
Related Services
Overview
WuXi AppTec DMPK can provide MIST research services and guide analytical decisions in a progressive manner based on experimental results. Our team has over 15 years of industry experience and includes dozens of experienced metabolic researchers. We have established a state-of-the-art platform equipped with various models of high-resolution mass spectrometry, including the Orbitrap Eclipse™ Tribrid™, Orbitrap Exploris™ 480, Q Exactive™ (QE), Q Exactive™ PLUS, Q Exactive™ HF, and VION™ IMS Qtof and other models of high-resolution mass spectrometry. Our research services cover the drug discovery stage from lead compound optimization selection to drug clinical research stage, as well as metabolic product analysis and identification using radioactive isotope tracing. We have supported the completion of over a thousand global application projects, with nearly a hundred clinical metabolite identification projects. We can complete thousands of metabolite identification studies annually.
Learn More


Assays
Study Strategies
After the drug enters the human body, the produced metabolites are all exogenous substances for the human body. When the exposure to a metabolite at a steady state exceeds 10% of the total exposures of all drug-related components in human, the safety of this metabolite should be evaluated in toxicological studies. Drug metabolites in plasma can be searched and identified using high-resolution mass spectrometry combined with relevant data processing software, as well as the relative abundances of the detected metabolites. For major metabolites, further comparison of its exposure to human and toxicological species should be determined. Results from the study can help to evaluate if the selected toxicological species are appropriate.

Case Study
-
A pooled plasma sample from human after repeated administration of a drug candidate is prepared by an AUC method, and then analyzed by LC-UV/HRMS. As shown in Figure 1 and Table 1, five metabolites are detected, identified and semi-quantitatively determined. Both M4 and M5 account for more than 10% of the exposure of the total drug-related components in human at steady state. Due to no metabolite standards, the peak areas of the human metabolites in the AUC-pooled human and animal plasma samples are compared (Figure 2). Results from the analysis can confirm whether the exposure of high proportion metabolites in toxicological animal species is higher than in human. To avoid matrix interference, blank human and animal plasma were added to real animal and human plasma (Figure 2). The formula in Figure 2 was used to evaluate the exposure of metabolites in human and animal plasma.
Learn More
-

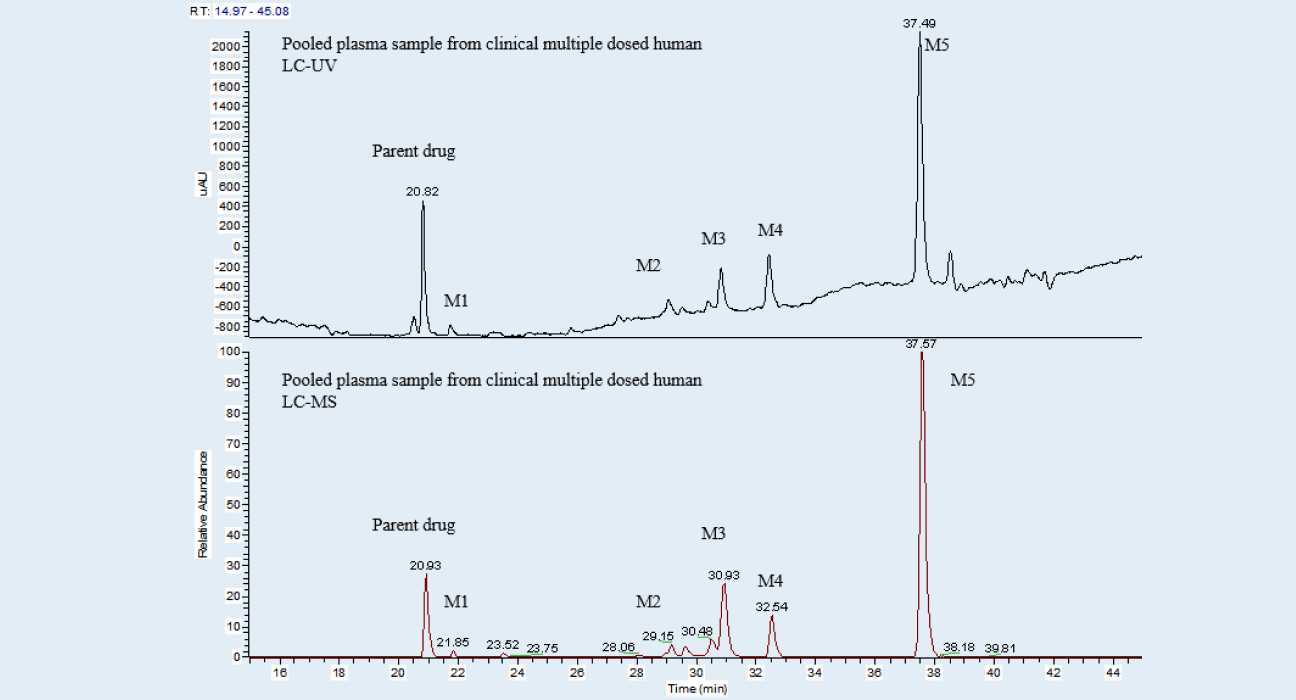
Metabolite profiles of parent drug and its metabolites in human plasma at steady state
Figure 1
-

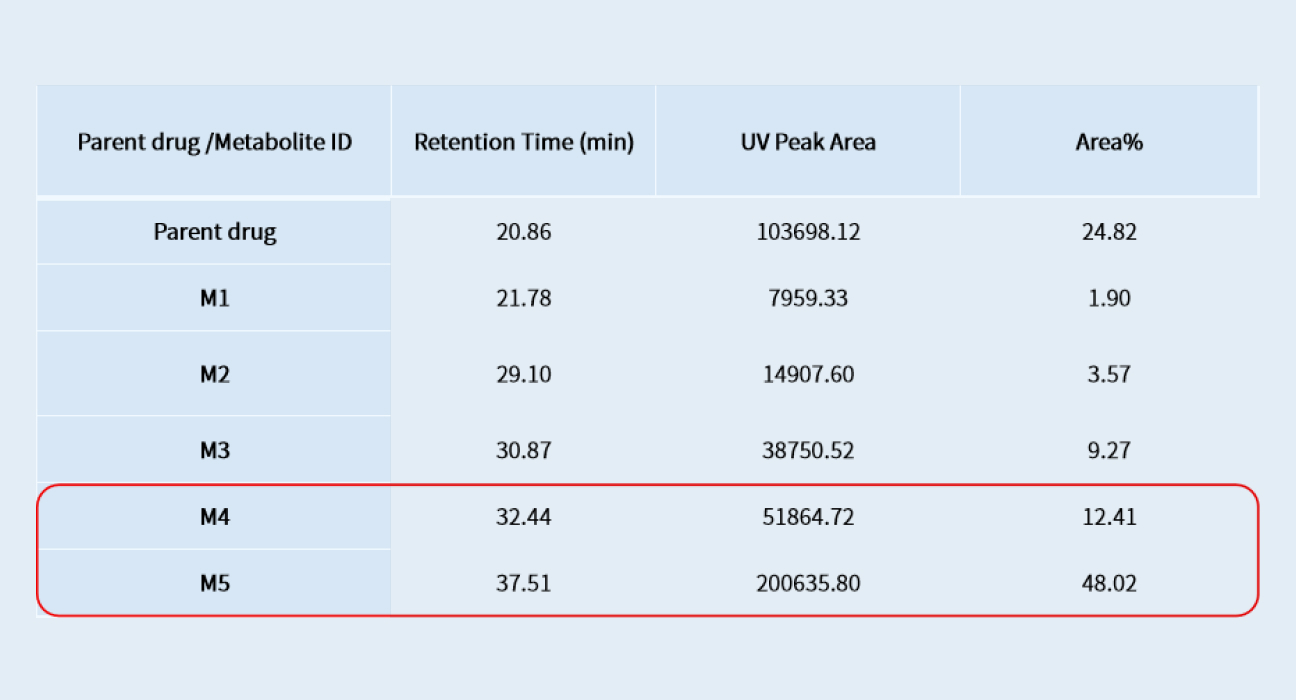
Relative abundance of parent drug and its metabolites in human plasma at steady state
Table 1
-

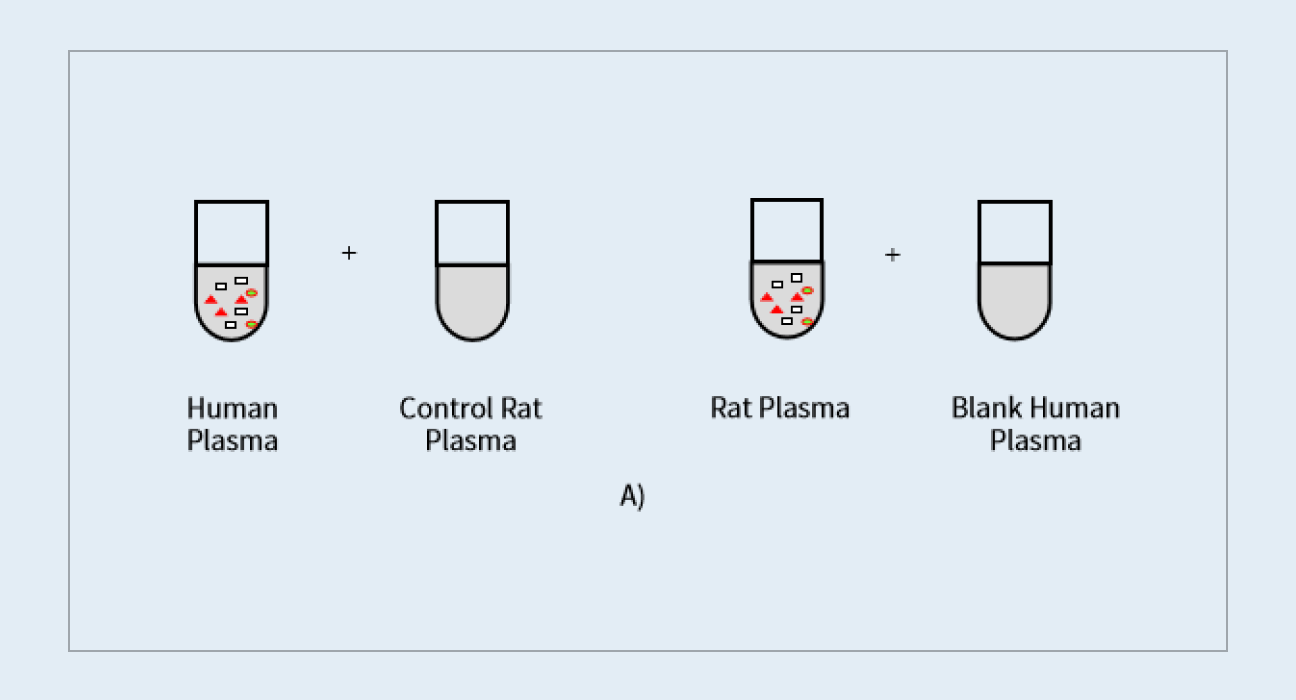
A) Mixed method to overcome matrix effect
Figure 2
-

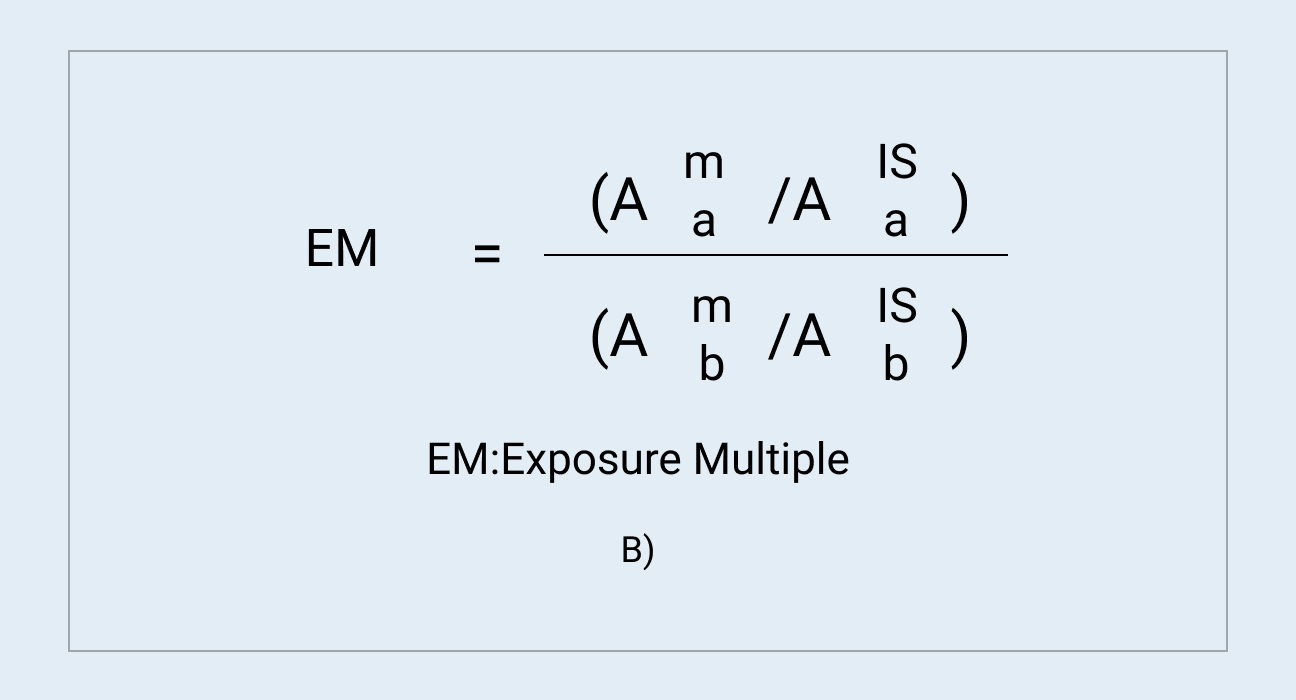
B) Formula for calculation of exposure multiple
Figure 2
-
Experience
-
10+
Years of experience
-
80%
Successfully supported NDA submissions for FDA and NMPA
-
10+
MIST studies
Instruments and Software
-
-


Thermo Orbitrap Eclipse™ Tribrid™
-


Thermo Orbitrap Exploris™ 480
-


Thermo Q-Exactive™ HF
-


Waters VION™ IMS QTof
-


Thermo Q-Exactive™
-


Thermo Q-Exactive™ Plus
-


Therno LTQ Orbitrap XL
-


Waters Xevo®G2 QTof
-


Solid scintillation counter: off-line detection of radioactivity
-


Liquid scintillation counter: total radioactivity detection
-


On-line detection of radioactivity
-
-
-


Thermo Scientific™ Compound Discoverer™
-


Thermo Scientific™ Mass Frontier™
-


Thermo Scientific™ Metworks™
-


Waters MetaboLynxTM
-


Mass Analytical Mass-MetaSite
-


Waters UNIFI®
-


Thermo Scientific™ Biopharma Finder™
-
FAQs
Related Resources




-


A Review on ADC Linkers: Cleavable and Non-Cleavable Linker Design and Stability Evaluation
ArticlesJan 30, 2026Learn More -


Software-aided Detection and Structural Characterization of Cyclic Peptide Metabolites in Biological Matrix by High-resolution Mass Spectrometry
PublicationsJan 09, 2026Learn More -


Practical Derivatization Approaches for LC‑HRMS Metabolite Profiling
ArticlesDec 30, 2025Learn More -


High-Resolution Mass Spectrometry-Based Data Acquisition and Data-Mining Technologies for Detecting and Characterizing Drug Metabolites and Traditional Chinese Medicine Components
PublicationsDec 11, 2025Learn More -


What Are Antibody–Oligonucleotide Conjugates (AOCs) and Their Structural Characteristics
ArticlesNov 28, 2025Learn More -


Overcoming Challenges in Oligonucleotide Metabolism with Innovative Solutions -WuXi AppTec DMPK TechTalk
VideosNov 07, 2025Learn More -


Rapid Screening and Characterization of Payload-Related Catabolites Derived from ADCs in In Vitro Models by UPLC-UV-HRMS
PostersOct 22, 2025Learn More -


Optimisation of an In Vitro Plated Monkey Hepatocyte Model and Comparative Metabolite Profiling of a GalNAc-Conjugated siRNA, siRNA01, in Monkey Liver Homogenate and Hepatocytes
PostersSep 26, 2025Learn More -


Probing Drug and Metabolite-Covalent Binding with a Non-Radiolabeled Workflow: Osimertinib as an Example
PostersAug 15, 2025Learn More -


Precision Metabolite Identification & Biotransformation for Drug R&D-WuXi AppTec DMPK
VideosJul 24, 2025Learn More -


New Book Release: Drug Metabolism and Pharmacokinetics: Frontiers, Strategies, and Applications | WuXi AppTec DMPK
VideosJul 17, 2025Learn More -


Decoding DMPK Frontiers for Novel Therapeutics: WuXi AppTec DMPK’s New Book Release
BlogsJul 10, 2025Learn More -


Drug Metabolism and Pharmacokinetics: Frontiers, Strategies, and Applications
PublicationsJun 13, 2025Learn More -


Unlocking Reliable In Vitro Metabolic Models for Oligonucleotide Therapeutics with the Latest Publication on DMD
BlogsMay 28, 2025Learn More -


High-Performance, Professional, Innovative: WuXi AppTec DMPK Accelerates Breakthroughs
VideosMay 22, 2025Learn More -


Comparative Metabolism of a GalNAc-Conjugated siRNA, Inclisiran, Among Various In Vitro Systems and Correlations with In Vivo Metabolism in Rats
PublicationsMay 08, 2025Learn More -


Detection and Characterization of In Vitro Payload-Containing Catabolites of Noncleavable Antibody-Drug Conjugates by High-Resolution Mass Spectrometry and Multiple Data Mining Tools
PublicationsApr 28, 2025Learn More -


Covalent Drugs DMPK Services
BrochuresApr 10, 2025Learn More -


Unlocking Precision in Peptide Metabolite Identification for Next-Gen Drug Development
ArticlesMar 19, 2025Learn More -


Insights into Chiral Drug Metabolism and Inversion
ArticlesFeb 13, 2025Learn More -


Methodologies and Strategies for ADC Biotransformation Studies
WebinarsJan 15, 2025Learn More -


WuXi AppTec DMPK: Your Trustworthy Partner
VideosDec 27, 2024Learn More -


Metabolite Identification in Peptide Drugs and Its Challenges
ArticlesDec 02, 2024Learn More -


Comparative Metabolite Profiling and Identification of a GalNAc-Conjugated siRNA, siRNA01, in Plasma Prepared with Various Anticoagulants, Serum, and In Vivo Plasma Using LC-UV-HRMS
PostersOct 25, 2024Learn More -


Overcoming Challenges in Precise Structural Identification of Target Metabolites
WebinarsOct 12, 2024Learn More -


Involvement of Aldehyde Oxidase (AOXs) in Drug Metabolism: Early Prediction and Coping Strategies
ArticlesSep 26, 2024Learn More -


Prodrug Approaches for Improved DMPK Characteristics and Their Preclinical Study Considerations
ArticlesAug 29, 2024Learn More -


An Analytical Workflow for Polymer Metabolism: PEGylated Lipids Biotransformation with LC-HRMS
PostersJun 27, 2024Learn More -


Development of a Convenient In Vitro Method for Predicting Metabolism and Disposition of Acrylamide Covalent Drugs in Humans
PostersMar 21, 2024Learn More -


Effect of pH on Metabolite Profiling and Identification of GalNAc Conjugated siRNA in In Vitro Metabolic System
PostersJan 23, 2024Learn More -


Metabolite Profiling and Identification of Oligonucleotide in In Vitro Metabolic System
PostersOct 30, 2023Learn More -


DAR Distribution Determination for Antibody-drug-conjugates (ADCs) by LC-HRMS in In Vitro and In Vivo Studies
PostersOct 27, 2023Learn More -


Drug Metabolism and Pharmacokinetics (DMPK) Service
BrochuresOct 27, 2023Learn More -


One-stop Metabolite Biosynthesis and Structural Characterization
BrochuresOct 27, 2023Learn More -


Overcoming the Challenges of Metabolite Identification and Profiling for Developing Oligonucleotides
BlogsOct 20, 2023Learn More -


Metabolite Profiling & Identification (MetID) Services
BrochuresOct 19, 2023Learn More -


Oligonucleotide Drugs: Strategies for Metabolism and Metabolite Profiling and Identification
ArticlesSep 13, 2023Learn More -


In Vitro-In Vivo Metabolite Profiling and Identification of Oligonucleotide
PostersAug 30, 2023Learn More -


A New Method to Improve Identification of the Payload-Containing Catabolites of ADCs
BlogsAug 10, 2023Learn More -


How to Address the Challenges of PROTAC Metabolism
BlogsJul 13, 2023Learn More -


How MetID Studies can Improve Safety and Efficacy in PROTAC Drugs
ArticlesJul 07, 2023Learn More
Stay Connected
Keep up with the latest news and insights.