-
Overview
-
Study Models and Platforms
-
Experience
-
Study Strategies and Assays
-
Case Study
-
FAQs
-
Related Resources
-
Related Services
Therapeutic Areas
Overview
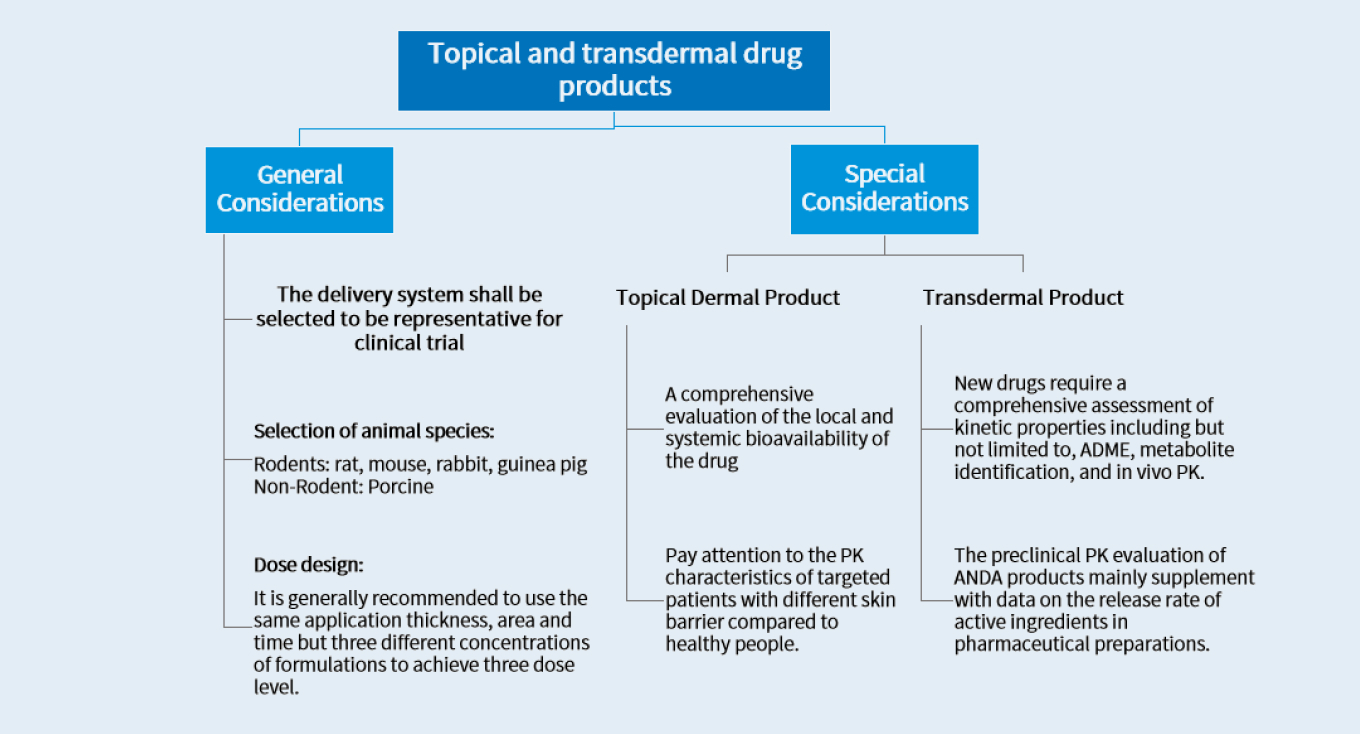
Topical and transdermal drugs have advantages such as good patient compliance, less adverse effects, and sustained release. They are now widely used in various therapeutic areas. Dermal administration imposes high demands on drug formulation development and dosage control. Precisely testing the in vitro release, permeation rate of drug formulations, and drug exposure in the system and skin components is crucial for evaluating the pharmacokinetic characteristics of drugs.
Learn More


Study Models and Platforms
-
In Vitro Release Testing (IVRT) and In Vitro Permeation Testing (IVPT) experiments
In vitro experiments are used to evaluate skin permeability, including drug release of different formulations and assessment of transdermal formulations by using biological skin or synthetic membranes. This approach partially reflects the clinical effectiveness of formulations and aids in investigating into factors affecting transdermal drug absorption. The Franz diffusion cell is used as the industry gold standard for dermato-pharmacokinetics 1. WuXi AppTec DMPK has successfully established IVRT and IVPT testing platforms.

-
In vivo techniques for evaluating the penetration of drugs into skin
The platform provides dermal administration of a wide range of drug formulation types for various animal species (mice, rats, minipigs, etc.), such as gels, creams, lotions, foams, patches, nanoparticles, and microneedles.

-
Collectable sample type
Types of skin tissue can be collected in the rat and minipig including stratum, epidermis, dermis, subcutaneous tissue, and muscle. Multiple sampling from a single minipig can be achieved by puncturing. For microneedle administration, the drug residue and recovery rate can be evaluated by microscopic examination.

-
Well-established models to mimic the barrier condition of different skin disease
To mimic the barrier condition of different skin diseases, we use professional, validated tools to create various models while ensuring animal welfare. The degree of skin damage is evaluated according to WuXi AppTec DMPK standards to ensure experiment reliability. Hematoxylin and eosin (H&E) staining of Bama miniature pig skin reveals a weakened barrier function after removing the epidermis, to enhance the permeability of drug into the skins.

Experience
-
5+
Years of preclinical research and development experience
-
15+
Transdermal formulations
-
2,000+
IVPT, and in vivo PK experiments in animals
Study Strategies and Assays
-
Topical and Transdermal Drug DMPK Research Strategies
The preclinical development of topical and transdermal drugs should adhere to non-clinical pharmacokinetic guidance. Unlike systemically administered drugs, these delivery systems have distinct characteristics in preion composition, dosage form, and administration route. Purposively designed strategies will expedite the drug development process.
Research Project | Topical Administration Route | Transdermal Administration Route | |
In Vitro Plasma Protein Binding Assay | Recommended if there is systemic exposure | Recommended | |
In Vitro Cell based Permeation and Inhibition Assay | |||
In Vitro Substrate and Inhibition of Transporters Assay | |||
In Vitro metabolic Stability Assay | |||
In Vitro metabolic Induction and Inhibition Assay | |||
In Vitro cytochrome p450 reaction phenotyping ** | |||
In Vivo Absorption | Species Choice | Two species are recommended: Mini pigs and one species of rodents | Two species based on the results of in vitro metabolite identification data for small molecular. Typically, choose species related to efficacy for macromolecules. * |
Sample Type | stratum corneum, epidermis, dermis, subcutaneous tissue, muscle, plasma | Plasma | |
Sample Quantity Time-Point Design | 3 samples/gender/time-point | 3 samples/gender/time point | |
No less than 5. | 10–12, typically 13–15 for macromolecules | ||
Administration Frequency | Single dose and multiple doses | Single dose and multiple doses | |
In Vivo Tissue Distribution | Recommended if there is systemic exposure | Recommend radioactive labeling experiment | |
In Vivo Excretion | Recommended if there is systemic exposure | Recommend radioactive labeling experiment | |
Metabolite Identification In Vivo (Skin or Plasma) | Recommended if there is systemic exposure | Recommend radioactive labeling experiment | |
Case Study
-
Commercial Compound A, a widely used anti-inflammatory nonsteroidal drug with rapid absorption and a short half-life, was used as a model drug. Validation experiments were conducted in a vertical Franz diffusion system to evaluate the in vitro transdermal character of six formulations, which included a clear solution formulation (Group 1), a commercial cream formulation (Group 2), and self-made poloxamer formulations (Group 3-6).
Before the experiment, Bama mini pig back skin was selected and its thickness was unified. The proceeded skin was placed between the donor and receptor chambers with the stratum corneum facing up. Dermal tissues with qualified TEWL were selected for the experiment, and a single dose of Compound A formulation was applied. Then, the temperature was set same as normal skin, and samples from the receptor chamber were collected at planned time points. After the experiment, the tissue was washed, and stratum corneum, the epidermis and dermis were peeled and collected to determine the distribution of the compound in the skin. The results are shown in Figures 1 and 2.
Figure 1 shows the commercial gel formulation (Group 2) significantly decreased the drug’s permeation flux and permeation amount compared to clear solution formulation (Group 1). For the four poloxamer formulations, different solvent ratios altered the drug’s transdermal characteristics. Figure 2 shows the residual amounts of Compound A in epidermis and dermis significantly decreased in four poloxamer formulations (Groups 3–6). These data help further screening and optimization of Compound A formulation and also show the vertical Franz diffusion system excellently discriminates the transdermal permeation ability of different formulations.
Learn More
-

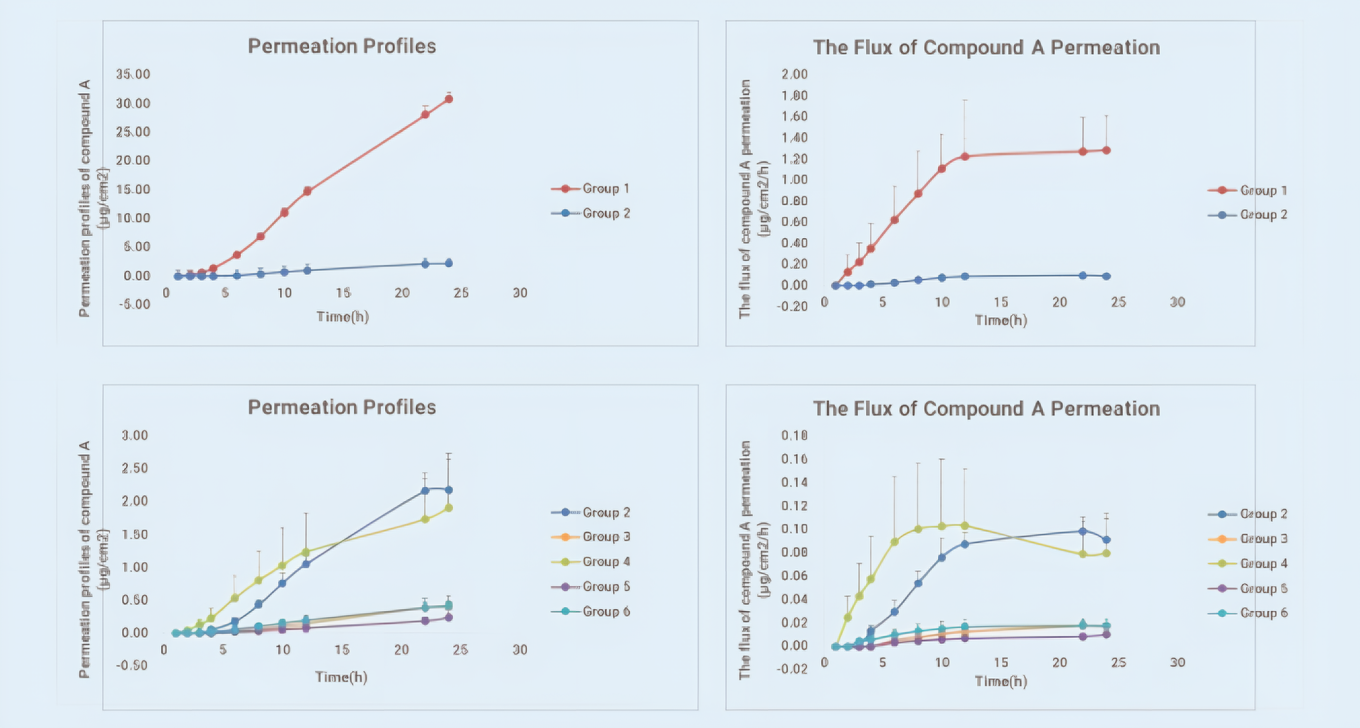
Cumulative permeation (left) and Percolation flux(right) -time curve of each formulation
Figure 1
-

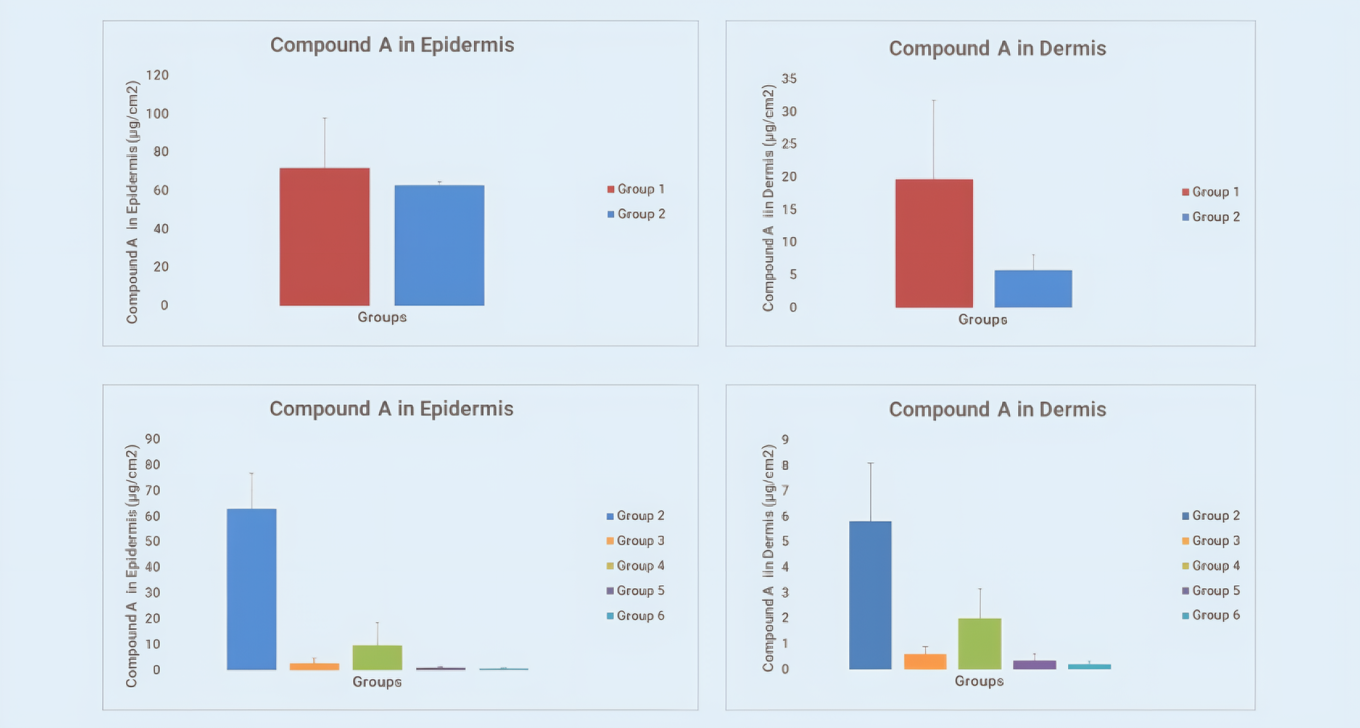
Residual amount of Compound A in the epidermis (left) and dermis (right) at 24 hours post-dose
Figure 2
-
FAQs
-
What’s the suggested LOQ of plasma in transdermal PK?
If there is very limited systemic exposure, 50-100 pg/mL LOQ is required.
-
Is it transdermal administration bio-availability is needed?
Yes, it is usually required well research on transdermal administration bio-availability.
-
Based on your experience, in minipig transdermal PK, could sampling in multiple timepoints contaminate blood and increase the concentration?
No. Clear the dose area strictly before collecting the skin tissue, it won’t contaminate blood or increase the blood concentration.
Related Resources




-


Topical and Transdermal Drugs DMPK Services
BrochuresJan 23, 2025Learn More -


Microneedling in Drug Delivery: Roles, Challenges, and Pharmacokinetic Strategies
ArticlesDec 23, 2024Learn More -


Evaluation of Different Tissue Processing Methods in Bama Pig Skin as an Animal Model for Topical Delivery Systems
PostersSep 19, 2024Learn More -


Application of In Vitro Permeation Test (IVPT) for the Development of Transdermal and Topical Drugs
ArticlesMay 15, 2024Learn More -


Transdermal Drug Delivery System (TDDS): Research Overview and Methods for Enhancing Skin Permeability
BlogsMar 29, 2024Learn More -


Evaluating In Vivo Pharmacokinetics for Transdermal Drugs Strategies and Methods
ArticlesMar 21, 2024Learn More -


In Vitro Release Testing Method in Semi-solid Topical Products Development
PostersJun 30, 2023Learn More -


Determination of Proxylane by LC-MS/MS Using Hypercarb Column
PostersJun 13, 2023Learn More -


In Vitro Evaluation of Transdermal Permeation and Skin Distribution of Different Diclofenac Formulations
PostersApr 28, 2023Learn More
References
- 1.
HEATHER A E. Transdermal and topical drug delivery: principles and practice. Hoboken: Wiley,2011.
- 2.
Migdadi EM, Courtenay AJ, Tekko IA, et al. J Control Release. 2018;285:142-151.
Stay Connected
Keep up with the latest news and insights.