-
Overview
-
Services
-
Featured Strengths
-
Instrument Platforms
-
FAQs
-
Related Resources
-
Related Services
DMPK BA
Overview
Since 2006, we have established a long-term mutual trust collaboration model with pharmaceutical companies worldwide. With an experienced professional analysis team, advanced analytical instruments, whole-process electronic management, and reliable quality control systems, we continuously empower our customers with high-efficiency and high-quality bioanalytical services from screening stages to a clinical trial application that includes drug discovery, in vitro and in vivo pharmacokinetics and pharmacodynamics. WuXi AppTec DMPK also provide high-quality customized services for the personalized needs of customers in large and small molecule drugs, biomarkers, immunogenicity, and cell-based assays.
Learn More


Services
-

Learn More
Small Molecules
Small molecule compounds, biomarkers, chiral compounds, metal containing compounds, and innovative drug delivery systems, etc.
-

Learn More
Novel Drug Modalities
Monoclonal antibodies (mAb), bispecific antibodies (bsAb), recombinant proteins, biosimilars, fusion proteins, peptides, antibody drug conjugates (ADC), Proteolysis Targeting Chimera (PROTACs*), peptides and proteins, hormones, oligonucleotides, gene therapy products and vaccines, etc.
Featured Strengths
-


Seamless Upstream and Downstream Integration
From screening to IND application
One-stop full-process analysis service
-


Comprehensive Service Types
Molecular weight range: 50 Da to 200K Da
100+ types of matrices
-


Advanced Instrument Platforms
Automated sample pre-treatment system
High-sensitivity, high-resolution analytical detection platform
High-throughput sample analysis platform
-


High-Quality Data
Dedicated instrument maintenance and quality control teams
Consistency verification across multiple sites, analytical platforms, and sample preparation methods
Compliance with IND application standards regulatory agencies such as NMPA, FDA, EMA, etc.
Instrument Platforms
-
-


Waters Vion™ QTof
-


Orbitrap Eclipse™ Tribrid™
-


Sciex Triple Quad™ 7500
-


PE NexION 2000
-
-
-


MESO Sector S 600MM
-


Molecular Devices SpectraMax M5e
-


BD LSRFortessa™ X-20
-
-
-


QIAcube HT
-


QuantStudio™ 7 Flex Real-Time PCR System
-


QIAgility
-
-
-


Apricot Designs Dual Arm
-


High-throughput intelligent delivery system
-
FAQs
-
What is the typical turnaround time (TAT) for a bioanalysis project?
The TAT for a DMPK bioanalysis project is the process from receiving the samples to uploading the analysis data. Generally speaking, the cycle for routine in vivo screening is 24-48 hours, and the cycle for in vitro screening is 24 hours. However, the specific TAT will be evaluated and appropriately adjusted by our analysis experts based on the actual situation.
-
What are LOD, LLOQ, and ULOQ?
LOD, LLOQ, and ULOQ are important parameters for ensuring the reliability of bioanalytical results, defined as follows:
LOD (Limit of Detection) refers to the lowest amount or concentration of analytes that can be reliably detected under certain analysis conditions but not necessarily for accurate quantification.
LLOQ (Lower Limit of Quantification) is the lowest concentration of analyte in a sample that can be reliably quantified with acceptable accuracy. The LLOQ is the lowest concentration point of the standard curve and should be suitable for the anticipated concentration and experiment purpose.
ULOQ (Upper Limit of Quantification) is the highest concentration of analyte in a sample that can be reliably quantified with acceptable accuracy under given analysis conditions and methods. The ULOQ is the highest concentration point of the standard curve and should be suitable for the anticipated concentration and experiment purpose.
-
What is method validation of bioanalysis?
Method validation in bioanalysis is a series of tests and evaluations to demonstrate the reliability of a specific analytical method in determining the concentration of an analyte in a certain biological matrix, aiming to prove that the adopted bioanalytical method is suitable for the intended purpose. Generally, the complete method validation of a chromatographic analysis should include selectivity, specificity, matrix effect, calibration curve (response function), range (from LLOQ to ULOQ), accuracy, precision, carryover, dilution linearity, stability, and reproducibility of sample re-injection, etc.
-
What are the advantages of conducting Non-GLP bioanalysis?
The advantage of Non-GLP bioanalysis is that it provides greater flexibility, allowing researchers to make necessary optimizations and adjustments during the experiment. Additionally, its relatively lower cost increases the cost-effectiveness of the research. Most importantly, Non-GLP bioanalysis can obtain experimental data quickly with a shorter turnaround time, thereby accelerating the research process.
-
What is bioanalysis in drug development?
In drug development, bioanalysis is the accurate measurement of drugs, metabolites, and biomarkers in biological matrices (e.g., plasma, tissues) to provide core data for decision‑making.
-
How do you ensure the accuracy and reliability of your results from bioanalytical services?
To ensure the accuracy and reliability of bioanalytical results, we employ a systematic quality management approach. This includes strict instrument calibration and lifecycle management for hardware and software, SOP‑driven processes, internal staff training, competency assessments, and oversight by independent quality control officers. Automated sample preparation and reporting systems combined with electronic trial records ensure timely, complete, and traceable data. Inter‑laboratory quality assessments and multi‑site/batch consistency evaluations validate data robustness. High‑standard instruments and facilities, together with an experienced team, ensure methods meet client and regulatory requirements, providing reliable data for research and development decisions.
Related Resources




-


Neonatal Fc Receptor (FcRn): Advances in Autoimmune Therapeutics and Analytical Strategies
ArticlesFeb 10, 2026Learn More -


Metabolomics: Definition, Analysis, and Applications
ArticlesFeb 05, 2026Learn More -


mRNA Therapeutics Quantification: RT-qPCR vs. bDNA Analytical Methods
ArticlesJan 23, 2026Learn More -


Practical Derivatization Approaches for LC‑HRMS Metabolite Profiling
ArticlesDec 30, 2025Learn More -


Rapid and Reproducible Dibutylation Derivatization Coupled with Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry for the Simultaneous Determination of Dopamine, Norepinephrine and 5-Hydroxytryptamine in Rat Brain Microdialysates
PublicationsDec 30, 2025Learn More -


Unveiling Immunogenicity: Precision Anti-Drug Antibody (ADA) Assays Empower Safer Biologic Drug Development | DMPK Frontiers
VideosDec 24, 2025Learn More -


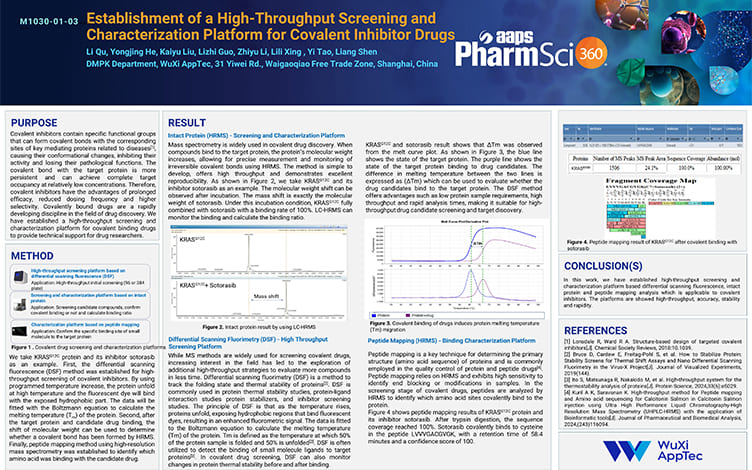
Establishment of a High-Throughput Screening and Characterization Platform for Covalent Inhibitor Drugs
PostersNov 21, 2025Learn More -


Unveiling Lipidomics Quantitative Analysis: What, Why, and How
ArticlesNov 13, 2025Learn More -


Integrated Bioanalytical Platform and DMPK Strategies for Antibody-Drug Conjugates
WebinarsNov 13, 2025Learn More -


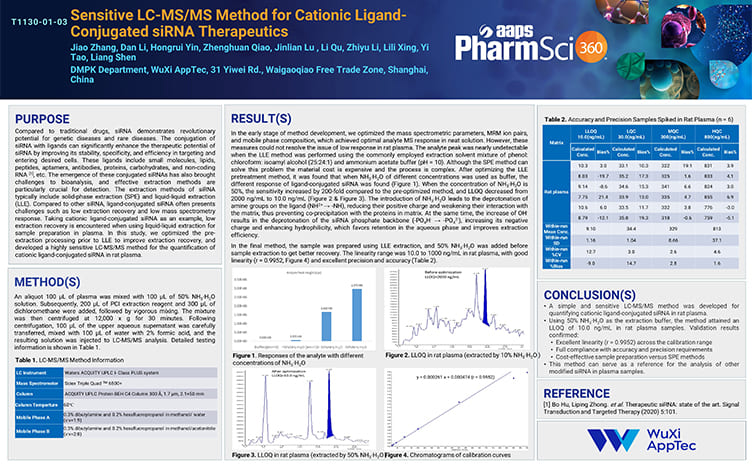
Sensitive LC-MS/MS Method for Cationic Ligand-Conjugated siRNA Therapeutics
PostersNov 08, 2025Learn More -


Biomarker Testing and Analysis: The Key to Accelerating Drug R&D
ArticlesOct 11, 2025Learn More -


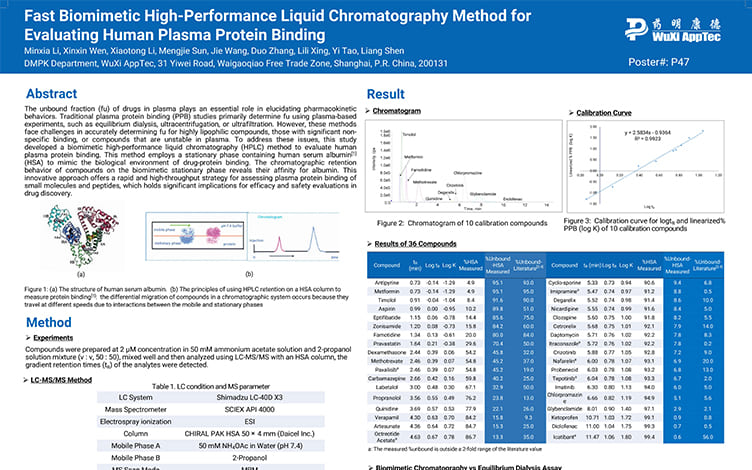
Fast Biomimetic High-Performance Liquid Chromatography Method for Evaluating Human Plasma Protein Binding
PostersSep 30, 2025Learn More -


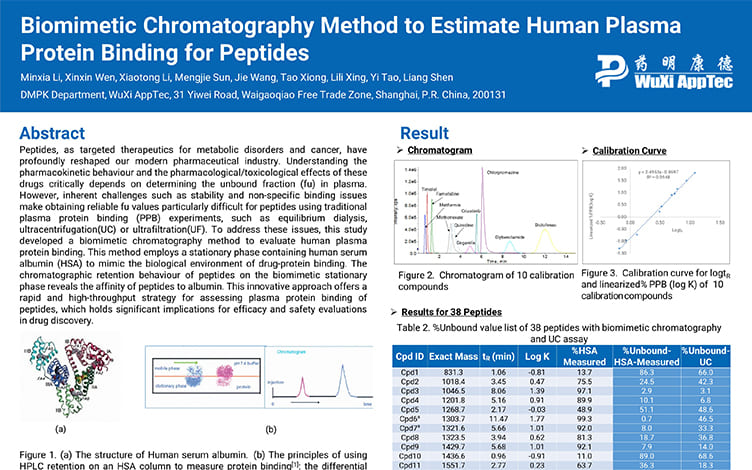
Biomimetic Chromatography Method to Estimate Human Plasma Protein Binding for Peptides
PostersSep 19, 2025Learn More -


Unveiling Amino Acid Analysis: Challenges and Best Practices with Biological Samples
ArticlesSep 11, 2025Learn More -


Bioanalytical Strategy for Antibody-Drug Conjugates (ADCs) Based on Integrated Mass Spectrometry Bioanalysis Platform
PostersSep 11, 2025Learn More -


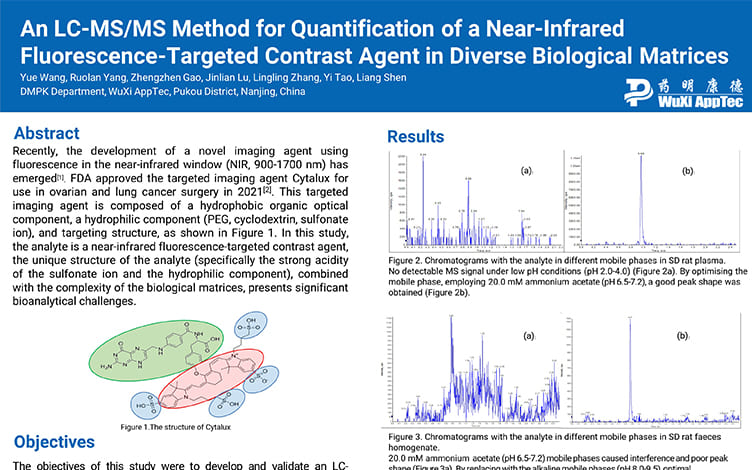
An LC-MS/MS Method for Quantification of a Near-Infrared Fluorescence-Targeted Contrast Agent in Diverse Biological Matrices
PostersAug 25, 2025Learn More -


Advancing Novel Therapeutics with Innovative DMPK Strategies in Metabolism and Bioanalysis
WebinarsAug 22, 2025Learn More -


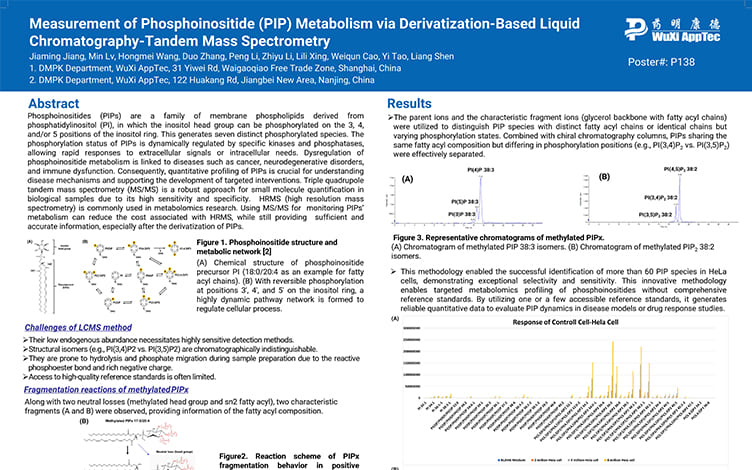
Measurement of Phosphoinositide (PIP) Metabolism via Derivatization-Based Liquid Chromatography-Tandem Mass Spectrometry
PostersAug 12, 2025Learn More -


An Efficient and Environmental Friendly Method for the Determination of Nucleoside Phosphate Compounds by Using LC-MS/MS
PostersAug 01, 2025Learn More -


Bioanalytical Strategy for Payload-Related Species of Antibody Drug Conjugates (ADCs) with LC-MS
PostersAug 01, 2025Learn More -


Development of a High-throughput and Cost-effective Method for Experimental Polar Surface Area Measurement with Ultra-Performance Convergence Chromatography Tandem Mass Spectrometry
PostersJul 24, 2025Learn More -


mRNA Vaccines and Therapeutics: Advances in Modification, Delivery, Applications, and Analytical Strategies
ArticlesJul 17, 2025Learn More -


New Book Release: Drug Metabolism and Pharmacokinetics: Frontiers, Strategies, and Applications | WuXi AppTec DMPK
VideosJul 17, 2025Learn More -


Optimization of Sample Pretreatment Methods for the Determination of an Oligonucleotide by Using LC-MS/MS
PostersJul 17, 2025Learn More -


Decoding DMPK Frontiers for Novel Therapeutics: WuXi AppTec DMPK’s New Book Release
BlogsJul 10, 2025Learn More -


An LC-MS/MS Method for Separation and Quantification of Chiral Molecules
PostersJul 10, 2025Learn More -


A Robust LC-MS/MS Method for the Detection of Covalent Drugs in Mouse Blood
PostersJul 10, 2025Learn More -


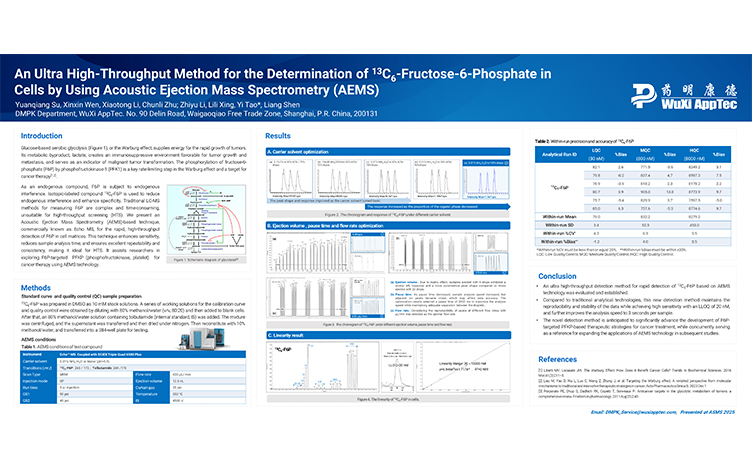
An Ultra High-Throughput Method for 13C6-Fructose-6-Phosphate in Cells Using Acoustic Ejection Mass Spectrometry (AEMS)
PostersJul 04, 2025Learn More -


A Novel Validated LC-MS/MS Method for the Determination of Survodutide in Mouse Liver
PostersJul 04, 2025Learn More -


Construction of a UPLC-UPC2-MS/MS Platform
PostersJun 27, 2025Learn More -


How to Analyze and Separate Oligonucleotides and Active Metabolites Using LC-MS/MS
ArticlesJun 20, 2025Learn More -


Drug Metabolism and Pharmacokinetics: Frontiers, Strategies, and Applications
PublicationsJun 13, 2025Learn More -


Development and Validation of a LC-MS/MS Method for Simultaneous Determination of TQ-A3326 and Its Major Metabolites in Human Plasma, Urine and Feces: Application to Pharmacokinetic Assay
PublicationsJun 12, 2025Learn More -


High-Performance, Professional, Innovative: WuXi AppTec DMPK Accelerates Breakthroughs
VideosMay 22, 2025Learn More -


Internal Standards in LC−MS Bioanalysis: Which, When, and How
ArticlesMay 15, 2025Learn More -


Immunogenicity Testing: Definition, Guidelines, and Anti-Drug Antibody Detection
ArticlesApr 25, 2025Learn More -


An Efficient Bioanalytical Method for mRNA Drug in Mouse Blood and Tissue Samples
PostersApr 17, 2025Learn More -


Covalent Drugs DMPK Services
BrochuresApr 10, 2025Learn More -


FAQs on Oligonucleotide DMPK Studies Stability, Distribution, Administration, and Bioanalysis
BlogsApr 03, 2025Learn More -


4β-Hydroxycholesterol as an Endogenous Biomarker of CYP3A Activity in Cynomolgus Monkeys
PublicationsMar 24, 2025Learn More -


Optimization of the LLOQ for Exatecan, a Widely-Used Antibody Drug Conjugate (ADC) Payload
PostersMar 12, 2025Learn More -


Bioanalytical Strategy for Quantitative Conjugated Payloads of Antibody-Drug Conjugates (ADCs) with Cleavable Linker
PostersMar 12, 2025Learn More -


Streamlining the Bioanalysis of Antibody-Drug Conjugates (ADCs): Simultaneous Determination of Total Antibodies and Conjugated Payloads
ArticlesMar 04, 2025Learn More -


Overview of Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Its Applications in Bioanalysis
ArticlesFeb 26, 2025Learn More -


Antibody-Drug Conjugate (ADC) Preclinical PK/PD Study Strategies and Practices
ArticlesFeb 19, 2025Learn More -


Simultaneous Determination of Metabolites from Seven Key Probe Substrates in CYP Inhibition Assay Using Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
PostersFeb 13, 2025Learn More -


Development of a Rapid, High Throughput Quantitative Bioanalysis Method for Conjugated Payloads of Protease-Cleavable Antibody-Drug Conjugate (ADC) in Rat Serum Using Automated Immuno-Capture Based UPLC-MS/MS
PostersFeb 06, 2025Learn More -


Comprehensive Mass Spectrometry Based Bioanalysis Strategy for Antibody-Drug Conjugate Therapeutics
PostersJan 30, 2025Learn More -


Unlocking High-Throughput Bioanalysis of Covalent Drugs with Intact Protein, DSF, and Peptide Mapping Techniques
ArticlesJan 20, 2025Learn More -


Exploring Our Latest Publication on Bioanalysis: Review on the Bioanalysis of Non-Virus-Based Gene Therapeutics
BlogsDec 30, 2024Learn More
Stay Connected
Keep up with the latest news and insights.