-
Overview
-
Permeability Assays
-
Transporter Assays
-
Case Studies
-
Agency/Organization Requirements
-
Experience
-
Factsheets
-
FAQs
-
Related Resources
-
Related Services
Overview
WuXi AppTec DMPK’s in vitro permeability and transporter platform provides a variety of models for permeability, transporter-mediated DDI, and hepatic uptake clearance assessment to meet the needs of high-throughput screening, mechanistic research, and application at different stages of drug development.
Learn More


Permeability Assays
Transporter Assays
-
MDR1-MDCK I /MDR1-MDCK II Cells
Assay types
substrate / inhibition assessment assays, Km & Vmax determination assays
Feature
To assess P-gp-mediated DDIs
To assess the transport mechanism for P-gp substrates
MDR1-MDCK I cells are mainly used to exclude P-gp substrates for CNS drug development
-
Caco-2 Cells
Assay types
substrate / inhibition assessment assays, Km & Vmax determination assays
Feature
To assess P-gp and BCRP-mediated DDIs
To assess the transport mechanism for P-gp and BCRP substrates
-
HEK-293 transfected Cells
Assay types
substrate / inhibition assessment assays, Km & Vmax determination assays
Feature
To assess DDIs for OATP1B1, OATP1B3, OATP2B1, OAT1, OAT3, OCT1, OCT2, MATE1, MATE2-K, PEPT1, PEPT2, and NTCP
To assess the transport mechanisms for substrates of OATP1B1, OATP1B3, OATP2B1, OAT1, OAT3, OCT1, OCT2, MATE1, MATE2-K, PEPT1, PEPT2, and NTCP
-
Vesicles
Assay types
substrate / inhibition assessment assays, Km & Vmax determination assays, and Ki determination assays
Feature
To assess DDIs for P-gp, BCRP, BSEP, and MRP1/2/3/4
To assess the transport and inhibition mechanisms for substrates and inhibitors of P-gp, BCRP, BSEP, and MRP1/2/3/4
May fail to identify highly permeable compounds or highly non-specific binding compounds as substrates
-
Primary Hepatocytes
Assay types
hepatic uptake and hepatic uptake clearance assays, Kp,uu determination assay
Feature
To overall predict hepatic clearance
To evaluate species differences using multiple species of suspension primary hepatocytes
Case Studies
-
-

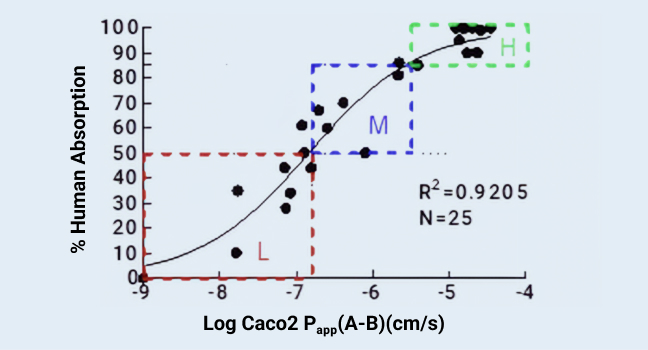
A correlation between human absorption versus estimates from Caco-2 logPapp(A-B) derived from a collection of 25 commercially available drugs
Figure 1
WuXi AppTec DMPK's in vitro permeability and transporter platform pays great attention to in vitro to in vivo correlations (IVIVCs) of in vitro models. For example, the IVIVC validation of Caco-2 permeability model was carried out using model drugs to define the binning criteria in 2015. Since then, the experiment has been repeated annually, and the results are reproducible. As shown in Figure 1, in the experiment conducted in 2024, the Papp (A-B) values of 25 commercially available drugs correlated very well with the human absorption data 2 (R2 of 0.9205).
-
-
-

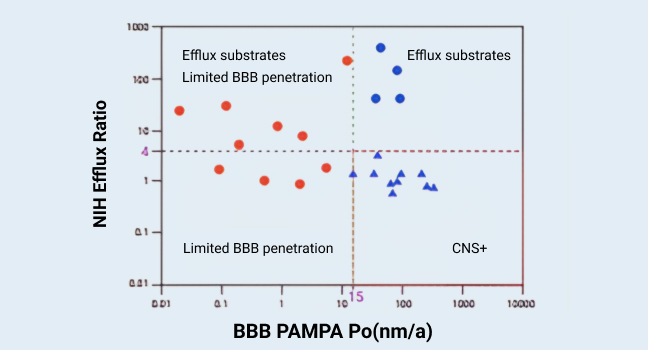
BBB penetration assessment for 24 marketed drugs
Figure 2
Brain penetration is one of the key factors for CNS drug development. WuXi AppTec DMPK's in vitro permeability and transporter platform developed a “Screening Funnel” model for brain penetration assessment. Ten “CNS+” drugs (high brain penetration, triangles) and 14 “CNS-” drugs (low brain penetration, dots) were included in the validation (Figure 2). All these drugs were tested in BBB-PAMPA, and ten “CNS-” drugs (red dots) were excluded due to limited BBB penetration. The others were tested in the MDR1-MDCK I (NIH) assay, and four “CNS-” drugs (blue dots) were further excluded due to high P-gp efflux. The remaining 10 compounds were potential “CNS+” (high brain penetration) compounds and were classified correctly.
-
Agency/Organization Requirements
| Transporter | Assay Type | Agency/Organization Requirement | |
| ABC | P-gp | MDR1-MDCK II, MDR1-MDCK I, Caco-2, and Vesicle‑MDR1 | FDA, EMA, NMPA, PMDA, and ICH |
| BCRP | Caco-2 and Vesicle-BCRP | ||
| BSEP | Vesicle-BSEP | EMA, PMDA, and ICH | |
MRP2 | Vesicle-MRP2 | PMDA and ICH | |
| MRP4 | Vesicle-MRP4 | PMDA | |
| MRP3 | Vesicle-MRP1 | Not mentioned | |
MRP1 | Vesicle-MRP1 | ||
| SLC | OATP1B1 | HEK293-OATP1B1 | FDA, EMA, NMPA, PMDA, and ICH |
| OATP1B3 | HEK293-OATP1B3 | ||
OAT1 | HEK293-OAT1 | ||
| OAT3 | HEK293-OAT3 | ||
| OCT2 | HEK293-OCT2 | ||
| MATE1 | HEK293-MATE1 | ||
| MATE2-K | HEK293-MATE2-K | ||
| OATP2B1 | HEK293-OATP2B1 | ICH | |
| OCT1 | HEK293-OCT1 | EMA, PMDA, and ICH | |
| PEPT1 | HEK293-PEPT1 | Not mentioned | |
| PEPT2 | HEK293-PEPT2 | ||
| NTCP | HEK293-NTCP | ||
Experience
-
17+
Years
-
40,000+
Compounds per year
-
1,500+
Studies for IND filing per year
FAQs
Related Resources




-


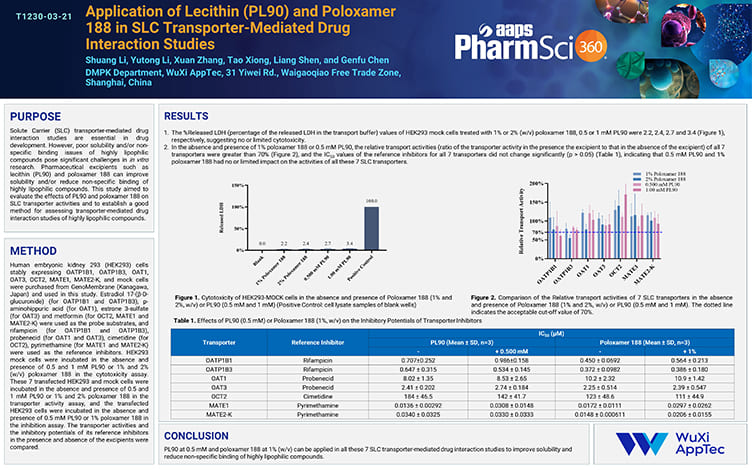
Application of Lecithin (PL90) and Poloxamer 188 in SLC Transporter-Mediated Drug Interaction Studies
PostersNov 21, 2025Learn More -


Establishment of a Novel Funnel Model for Evaluating Blood-Brain Barrier Penetration In Vitro
PostersSep 30, 2025Learn More -


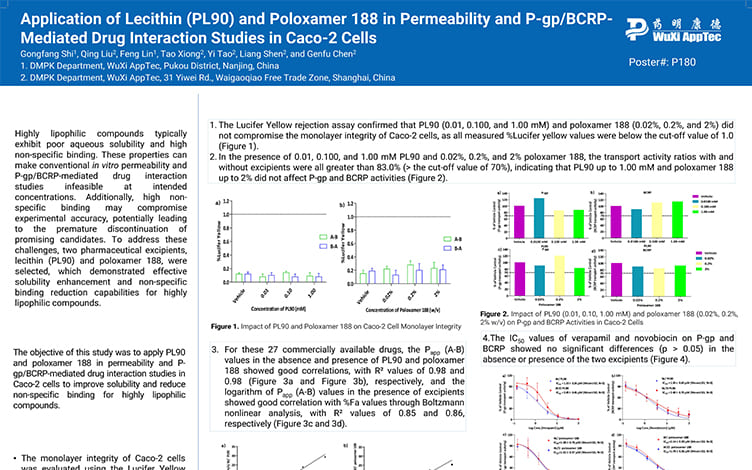
Application of Lecithin(PL90) and Poloxamer 188 in Permeability and P-gp/BCRP-Mediated Drug Interaction Studies in Caco-2 Cells
PostersJul 23, 2025Learn More -


Cyclic Peptides: FDA-Approved Drugs and Their Oral Bioavailability and Metabolic Stability Tactics
ArticlesMay 08, 2025Learn More -


Impact of Bovine Serum Albumin on In Vitro Permeability and ABC Transporter Mediated Drug Interaction Evaluation
PostersDec 03, 2024Learn More -


A Sensitive and Efficient LC-MS/MS Method for Creatinine-d5 Analysis in In Vitro OCT2 Inhibition Assay
PostersNov 28, 2024Learn More -


Permeability and Intestinal Absorption Study for Orally Administered Drugs: Preclinical Research Methods and Strategies
ArticlesSep 30, 2024Learn More -


The Role of Hepatic Transporters in Drug-induced Liver Injury (DILI) Research and Preclinical Evaluation Recommendations
ArticlesSep 13, 2024Learn More -


Application and Strategy of 3 In Vitro Models for ATP-binding Cassette (ABC) Transporters
ArticlesJul 19, 2024Learn More -


Permeability & Transporter Services Part Ⅰ: Permeability
BrochuresJun 14, 2024Learn More -


Permeability & Transporter Services Part Ⅱ: ABC Transporters
BrochuresJun 06, 2024Learn More -


Permeability & Transporter Services Part Ⅲ: SLC Transporters
BrochuresMay 31, 2024Learn More -


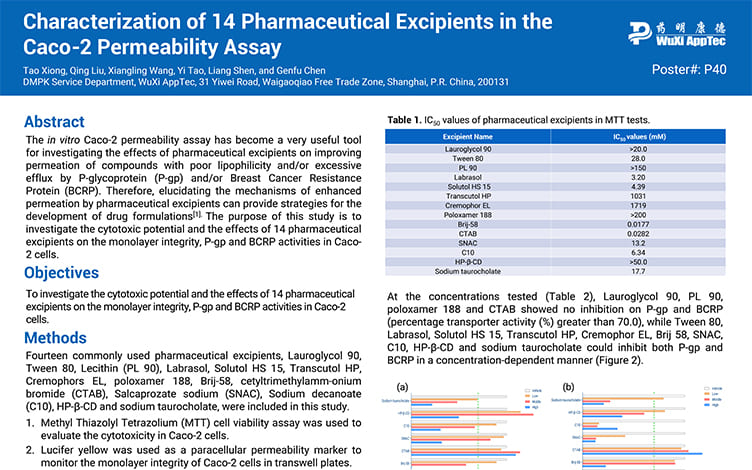
Characterization of 14 Pharmaceutical Excipients in the Caco-2 Permeability Assay
PostersMay 15, 2024Learn More -


Establishment and Characterization of an In vitro Model for NTCP Substrate and Inhibition Assessment
PostersMay 06, 2024Learn More -


An Ultra-high Throughput Bioanalysis Platform with Echo® MS for OCT1 and OCT2 Inhibition Assay
PostersJan 18, 2024Learn More -


Choosing Appropriate Model to Assess PROTAC Drug-Drug Interactions with Efflux Transporters
BlogsJul 26, 2023Learn More -


How to Evaluate PROTAC Drug-Drug Interactions with Efflux Transporters
ArticlesJul 07, 2023Learn More
References
- 1.
Giacomini, K.M. et al. Membrane transporters in drug development. Nature reviews drug discovery 9, 215-236 (2010)
- 2.
Suzanne, S. et al. Towards Prediction of in vivo intestinal absorption using a 96-well Caco-2 Assay. J. Pharm. Sci. 99, 32463265 (2010)
Stay Connected
Keep up with the latest news and insights.