-
Overview
-
Assays
-
Study Strategies
-
Case Study
-
Experience
-
Instruments
-
FAQs
-
Related Resources
-
Related Services
Overview
The radiolabeled study is a valuable and irreplaceable tool to elaborate drug ADME at drug discovery and development stages. Various species (mouse, rat, minipig, dog, and monkey) can be provided in our labs for in vivo ADME studies with radiolabeled compounds. The types of compounds include but not limited to regular small molecules, peptides, Proteolysis-Targeting Chimeras (PROTACs*), PDC, ADC, and oligonucleotides. Following administration of the radioactive drugs, samples are analyzed to determine the amount of radioactivity, structure of metabolites, and PK of parent and metabolites. The radiolabeled mass balance together with metabolic profiles in excreta make it possible to determine clearance mechanisms of drug candidates. The data of preclinical species also helps to define whether human metabolites have adequate exposure in toxicology species and if any metabolite contributes significantly to its pharmacological activity.
Learn More


Assays
Study Strategies
Generally, radiolabeled preclinical in vivo ADME studies are recommended to be completed in the IND phase or clinical phase I. In general, the sample collection time for mass balance of small animals is 168 h, while the sample collection time for bile excretion is 72 h. This time design can meet the recovery rate of more than 90% in most cases. For large animal mass balance experiments, we recommend sample testing immediately after collection to determine whether the collection time needs to be extended to ensure that the cumulative recovery rate of mass balance reaches 90%, and the total recovery rate in urine and feces for two consecutive days is less than 1%. Radioactive PK time points are generally designed to cover 3 to 5-time half-life of total compound–related materials. Quantitative tissue distribution studies are an essential component of ADME analysis. QWBA or tissue dissection methods can be chosen to deliver the information to understand the relationship between the distribution and accumulation of compounds and/or its metabolites, to support tissue-specific activity, and/or tissue-potential toxicity. Radiolabeled metabolite profiling and metabolite identification studies cover plasma and excreta such as bile, urine, feces, etc., to elucidate the main compound forms and relative exposures present in plasma and excreta, in combination with radioactive detectors and high-resolution mass spectrometry.
Case Study
-
We have well-established platform to use radiolabeled (14C or 3H) compounds to conduct the radiolabeled ADME studies in mouse, rat, dog, monkey and mini-pig. The recovery of 133 mass balance studies from 2020.01 to 2023.06 was summarized as below. In 95% of mass balance studies, the total recovery exceeded 90% of the administrated dose, The cumulative total recovery of 83% bile excretion (0-72 h post-dose) studies exceeded 90% of the administrated dose.
Learn More
-

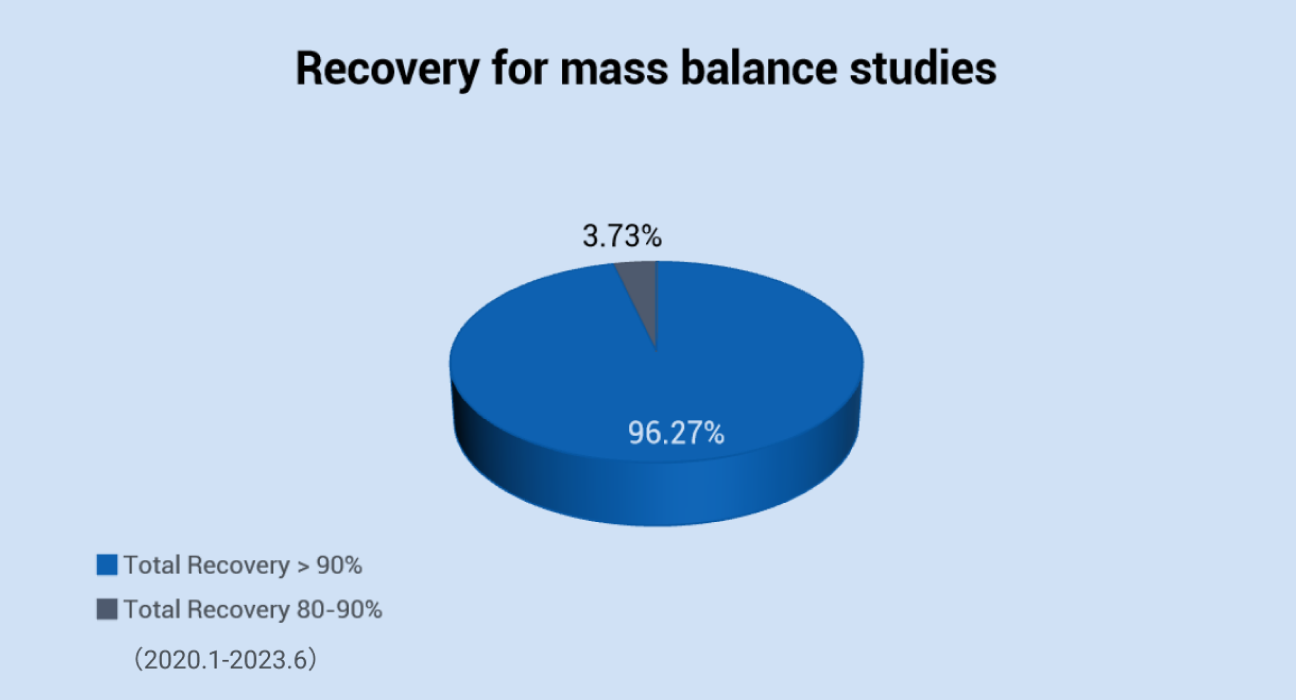
The recovery for mass balance studies
-

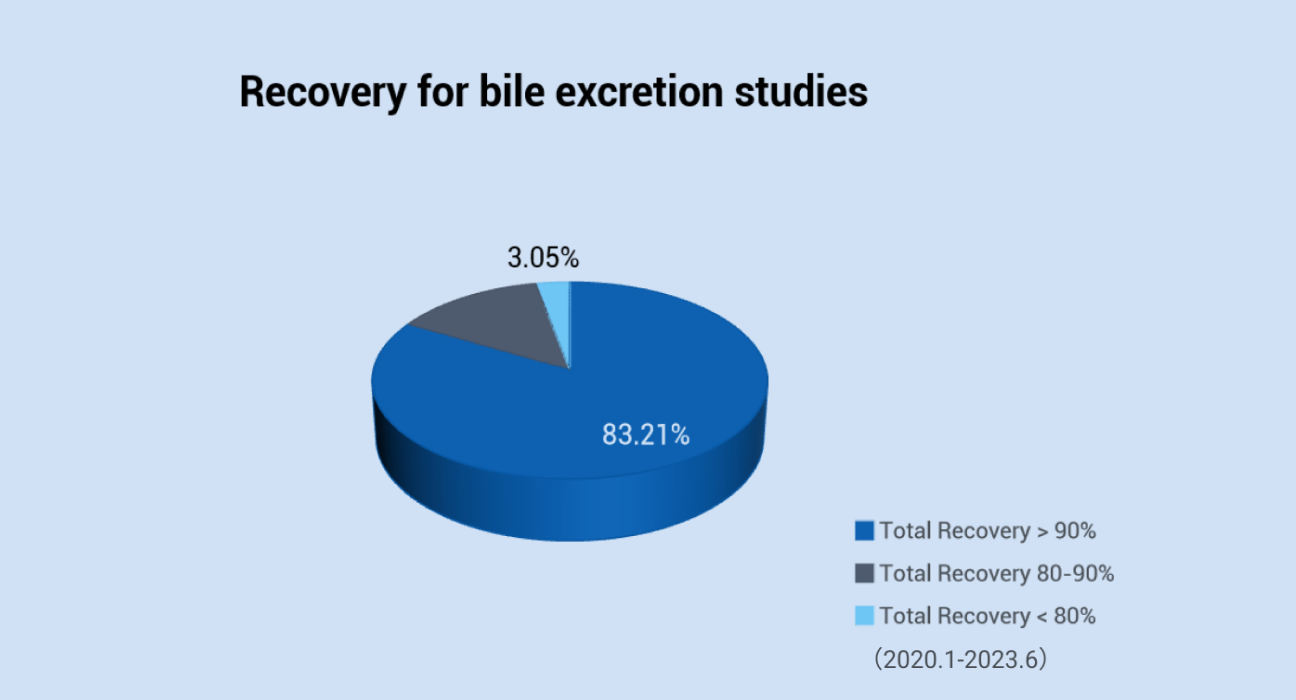
The recovery for bile excretion studies
-
Experience
-
15+
Years of practical experience
-
500+
Preclinical rodent and large animal in vivo ADME studies
-
Multiple compounds obtained FDA and/or NMPA NDA or marketing approval
Instruments
-
-

Liquid Scintillation Counter
-


β-RAM Online Radioactivity Detector
-


β-counter Solid Scintillation Counter
-

High-Resolution MS
-
FAQs
Related Resources




-


Metabolite Identification of Nucleoside Analog Prodrugs in Biological Matrix by Derivatization Coupled with Radio-Detector and Mass Spectrometry
PostersMar 07, 2024Learn More -


Radiolabeled Mass Balance Studies in Preclinical Animals
PostersFeb 27, 2024Learn More -


Using Radiolabeling Techniques to Improve ADC Pharmacokinetic Studies
BlogsAug 18, 2023Learn More -


Application of Radiolabeling Techniques in ADC PK Studies
ArticlesJul 26, 2023Learn More
Stay Connected
Keep up with the latest news and insights.





















