Peptide drugs are highly regarded for their high potency, specificity, low side effects, and lower production costs compared to antibody-based therapies. However, challenges such as poor metabolic stability, membrane permeability, and oral bioavailability—stemming from their large molecular weight, high polar surface area, and susceptibility to amide bond hydrolysis—have hindered their development. Recent advancements in phage display and mRNA display technologies have enabled rational design approaches, accelerating the discovery of peptide therapeutics.
Cyclic Peptide vs Linear Peptide
Peptide drugs are categorized into linear peptides and cyclic peptides. Cyclic peptides exhibit superior drug-like properties, including enhanced conformational rigidity, elimination of unstable terminal residues, intramolecular hydrogen bonding, lower polarity, higher target binding affinity, and enhanced metabolic stability compared to linear counterparts. These traits improve permeability, enabling oral administration or targeting intracellular sites. As of June 2024, 66 cyclic peptide drugs have been approved globally, with 39 gaining approval post-2000. In 2023, three of the six approved peptide drugs were cyclic: Rezafungin (for candidemia), Motixafortide (targeting CXCR4 in multiple myeloma), and Zilucoplan (a self-administered C5 inhibitor for generalized myasthenia gravis). According to a report by Research and Markets, the macrocyclic and stapled peptide market is projected to grow from 1.22 billion (2024) to 4.76 billion (2030), at a CAGR of 21.44%. Despite these advantages, cyclic peptides still face hurdles in metabolic stability and membrane permeability, which are critical focus areas for ongoing research.
Table 1. Cyclic Peptide vs Linear Peptide Properties
Property | Cyclic Peptides | Linear Peptides |
Conformation | Restricted | Flexible |
Terminal Residues | Absent or less | Present |
Hydrogen Bonds | Intramolecular | Intermolecular |
Polarity | Lower | Higher |
Affinity | Higher | Lower |
Stability | Higher | Lower |
Permeability | Higher | Lower |
Oral Administration | Feasible | Less Likely |
Intracellular Targets | Feasible | Less Likely |
Improving Cyclic Peptide Stability
Strategies to enhance stability include:
Backbone Modifications: Incorporating D-amino acids, non-canonical amino acids (e.g., β-/γ-amino acids), and N-methylation. Bioisosteric modifications could preserve pharmacophore geometry while enhancing metabolic stability.
Cyclization Optimization: Selecting optimal ring size and introducing double cyclization to reduce conformational flexibility and proteolytic degradation.
Secondary Structure Stabilization: Using α-helices, β-sheets, stapled peptides, or α/β-hybrid peptides.
Case Study: The natural cyclic peptide somatostatin (14 amino acids) has a short half-life (<3 minutes). Structural modifications—replacing L-phenylalanine and L-tryptophan with D-amino acids, converting the C-terminal carboxyl to an alcohol group—yielded octreotide, an 8-mer analog with a 100-minute half-life. Octreotide is now used to treat acromegaly and neuroendocrine tumors.
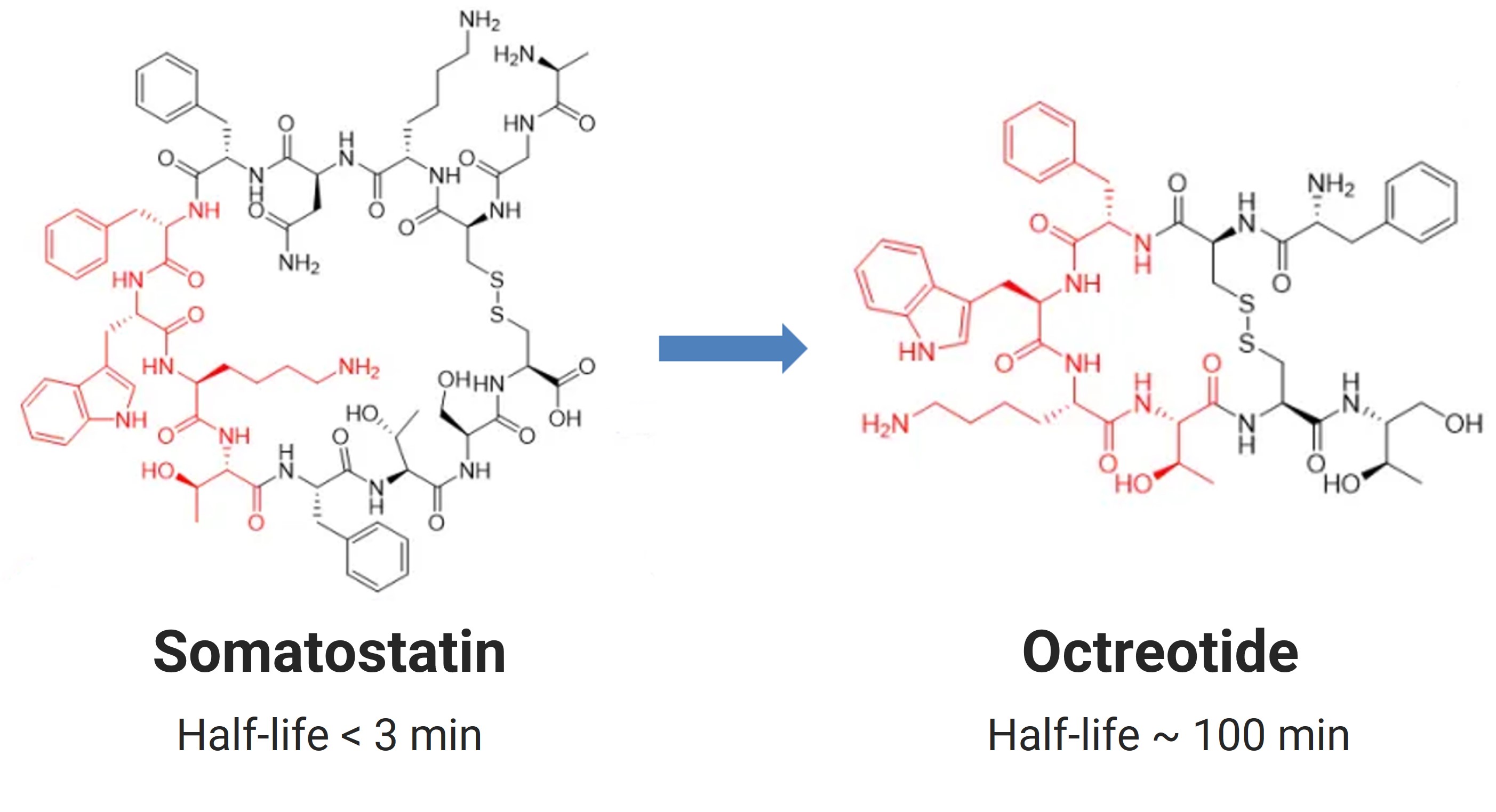
Figure 1. Modification of Somatostatin to Octreotide (red indicates the pharmacophore)
Double Cyclization: A urokinase plasminogen activator-binding peptide showed 40% remaining in murine plasma after 24 hours as a double-cyclized compound, outperforming linear and single-cyclized counterparts.

Figure 2. Linkage of Dual-Cyclic Peptides[3]
Enhancing Cyclic Peptide Membrane Permeability
Passive diffusion across biological membranes requires low molecular weight, lipophilicity, and minimal polar surface area. The "Expanded Rule of 5" (Table 2) guides oral small-molecule drug design but is often exceeded by cyclic peptides. For instance, cyclosporine A—a natural cyclic peptide with a molecular weight of 1,203 Da—achieves 20–70% oral bioavailability due to N-methylation, non-canonical amino acids, and a "chameleonic" ability to adopt lipid-compatible or aqueous conformations.
Table 2. Expanded Rule of 5 for Oral Drug Design
Property | Expanded Rule of 5 |
Molecular Weight | < 500 Da |
H-Bond Acceptors | < 10 |
H-Bond Donors | < 5 |
LogP | < 5 |
Rotatable Bonds | < 10 |
Polar Surface Area | < 140 Ų |
Recent studies propose "Beyond Rule of 5" guidelines for cyclic peptides (Table 3), emphasizing exposed polar surface area (EPSA) <90 Ų and CLogP 7.5–10 as critical predictors of permeability.

Figure 3. Correlation of EPSA and CLogP with MDCK Cell Permeability[5]
Table 3. Properties of Cyclic Peptide Drug Design Beyond the Rule of 5[6]
Property | Beyond the Rule of Five |
Molecular Weight | < 1000 Da |
Number of Hydrogen Bond Acceptors | < 15 |
Number of Hydrogen Bond Donors | < 6 |
LogP | 7.5-10 |
Number of Rotatable Bonds | < 20 |
Polar Surface Area | < 250 Ų |
EPSA | < 90 Ų |
N-methylation can modify the conformation, hydrogen bonding potential, and lipophilicity of cyclic peptides, enhancing their membrane permeability. For example, the Veber-Hirschmann peptide, a synthetic analogue of somatostatin, retains the active sequence of somatostatin and exhibits receptor selectivity. N-methylation modifications resulted in a new analogue that had improved Caco-2 permeability from 1 x 10-6 cm/s to 4 x 10-6 cm/s.
Another promising approach involves cell-penetrating peptides (CPPs), which were discovered in the early 1990s, with nearly 2000 peptides identified as having cell-penetrating activity. Cyclic CPPs demonstrate superior stability and cytoplasmic transport efficiency compared to linear CPPs. They interact directly with membrane phospholipids, entering cells via endocytosis or direct translocation. Therefore, they can serve as carriers for peptide drugs. Internal cyclization can couple linear peptides and small peptides with cyclic CPPs, while external and dual-cyclization coupling methods are suitable for larger peptides and cyclic peptides. For certain target proteins, peptide ligands must be in a linear form to exhibit biological activity, which is why reversible cyclization or dual-cyclization coupling methods have been designed to release linear peptides in the cytoplasm for targeted binding[8].

Figure 4. Cell-Penetrating Peptide 1 (CPP1)[8]
Development of Oral Cyclic Peptide Drugs
Cardiovascular disease is the leading cause of death globally, with hypercholesterolemia being a significant risk factor for atherosclerotic cardiovascular diseases (primarily coronary heart disease and ischemic stroke). Major lipid-lowering drugs include statins and fibrates, however, approximately 50% of individuals using statins fail to reduce their low-density lipoprotein cholesterol (LDL-C) to the desired level and face issues such as adverse effects, the need for combination therapy, and long-term use. Recently, PCSK9 inhibitors have emerged as a new treatment option for patients with various forms of difficult-to-treat hypercholesterolemia, regulating blood lipids by degrading LDL-C. Currently, three PCSK9 inhibitors are available globally: Evolocumab and Alirocumab (both monoclonal antibodies) and Inclisiran (an siRNA drug), demonstrating safety and efficacy that surpass statins. Evolocumab achieved sales of $1.296 billion in 2022, and the other two drugs are expected to become billion-dollar blockbusters. However, these three drugs also have drawbacks, such as poor patient compliance with subcutaneous injections and high costs, making the development of new oral PCSK9 inhibitors a hot topic.
MK-0616 is an oral cyclic peptide PCSK9 inhibitor for treating adult hypercholesterolemia. In August 2023, it entered Phase III clinical trials, with the potential to become the first oral PCSK9 inhibitor. The development process began with the discovery of hit peptide compounds through mRNA display technology, followed by optimization based on structural drug design. Compound 44 was found to be susceptible to chemical oxidation due to sulfide incorporation during macrocyclization. Key modifications included replacing the thiol linker and central triazole (from red to green) to minimize oxidation sensitivity (44 = 98%, MK-0616 = 2.9%) while maintaining potency (44 Ki = 2 pM, MK-0616 Ki = 5 pM) and changing the alkene to an amide cross-linker to increase solubility by reducing lipophilicity and avoiding isomerization from alkene formation[9].

Figure 5. Structural Optimization of Lead Compound 44 to MK-0616 [9]
In passive permeability assessments, MK-0616 exhibited very low transcellular permeability (Papp = 0.71 x 10^-6 cm/s). The clinical trial formulation used sodium caprate or Labrasol as permeation enhancers to increase intestinal absorption. At a 200 mg dose, the formulation containing Labrasol showed a 5-fold increase in Cmax (45.3 nmol/L vs. 7.84 nmol/L) and a 2-fold increase in AUC0-inf (1250 h•nmol/L vs. 556 h•nmol/L) compared to the formulation without Labrasol. At a 100 mg dose, the exposure levels with Labrasol and sodium caprate were similar, with a bioavailability of approximately 2%. Across a dose range of 10 to 300 mg, AUC0-inf showed less than linear growth, with half-lives ranging from 35 to around 130 hours.
After a high-fat breakfast (55.6 g fat, 55 g carbohydrates, 31.1 g protein), administering a 40 mg dose of MK-0616 resulted in geometric mean ratios of AUC0-inf and Cmax compared to fasting conditions of 0.33 and 0.25, respectively, indicating that food conditions adversely affect oral absorption.

Figure 6. Blood Concentration Curve After Single Dose of MK-0616[9]
In Phase II clinical trials, MK-0616 demonstrated placebo-adjusted reductions in LDL-C after eight weeks of once-daily administration, with a statistically significant and robust dose-dependent reduction of up to 60.9% from baseline, showing good tolerability during both the eight-week treatment and an additional eight-week follow-up[10].
Two Phase III clinical trials for MK-0616 were initiated in 2023, with the potential for successful market entry as a blockbuster drug due to its excellent efficacy, safety, and convenience, with industry projections suggesting peak annual sales could reach $5 billion. The development path and clinical results of MK-0616 provide direction for the research and development of macrocyclic peptides. By combining mRNA display technology, structure-based modifications to enhance stability and solubility, and using permeation enhancers, macrocyclic peptides that are beyond the Rule of Five can achieve viable oral bioavailability.
The Bottom Line
Cyclic peptides represent a transformative class of therapeutics, combining the advantages of small molecules and biologics. Innovations in cyclization strategies, backbone engineering, and formulation technologies are addressing historical limitations in metabolic stability and membrane permeability. With hundreds of cyclic peptide candidates in clinical trials for oncology, infectious diseases, and metabolic disorders, this field is poised to redefine therapeutic paradigms.
Authors: Jianping Sun, Liping Ma, Jing Jin
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] You, Sophia, Glen McIntyre, and Toby Passioura. “The Coming of Age of Cyclic Peptide Drugs: An Update on Discovery Technologies.” Expert Opinion on Drug Discovery 19, no. 8 (August 2024): 961–73. http://doi.org/10.1080/17460441.2024.2367024.
[2] Brian Chia, C.S. “A Review on the Metabolism of 25 Peptide Drugs.” International Journal of Peptide Research and Therapeutics 27, no. 2 (June 2021): 1397–1418. http://doi.org/10.1007/s10989-021-10177-0.
[3] Khatri, Bhavesh, Venkateswara Rao Nuthakki, and Jayanta Chatterjee. “Strategies to Enhance Metabolic Stabilities.” Methods in Molecular Biology (Clifton, N.J.) 2001 (2019): 17–40. http://doi.org/10.1007/978-1-4939-9504-2_2.
[4] Mathiowetz, Alan M. “Design Principles for Intestinal Permeability of Cyclic Peptides.” In Cyclic Peptide Design, edited by Gilles Goetz, 1–15. New York, NY: Springer, 2019. http://doi.org/10.1007/978-1-4939-9504-2_1.
[5] Boehm, Markus, Kevin Beaumont, Rhys Jones, Amit S. Kalgutkar, Liying Zhang, Karen Atkinson, Guoyun Bai, et al. “Discovery of Potent and Orally Bioavailable Macrocyclic Peptide–Peptoid Hybrid CXCR7 Modulators.” Journal of Medicinal Chemistry 60, no. 23 (December 14, 2017): 9653–63. http://doi.org/10.1021/acs.jmedchem.7b01028.
[6] Poongavanam, Vasanthanathan, Bradley C. Doak, and Jan Kihlberg. “Opportunities and Guidelines for Discovery of Orally Absorbed Drugs beyond Rule of 5 Space.” Current Opinion in Chemical Biology 44 (June 2018): 23–29. http://doi.org/10.1016/j.cbpa.2018.05.010.
[7] Buckton, Laura K., Marwa N. Rahimi, and Shelli R. McAlpine. “Cyclic Peptides as Drugs for Intracellular Targets: The Next Frontier in Peptide Therapeutic Development.” Chemistry – A European Journal 27, no. 5 (January 21, 2021): 1487–1513. http://doi.org/10.1002/chem.201905385.
[8] Appiah Kubi, George, Patrick G. Dougherty, and Dehua Pei. “Designing Cell-Permeable Macrocyclic Peptides.” In Cyclic Peptide Design, edited by Gilles Goetz, 2001:41–59. Methods in Molecular Biology. New York, NY: Springer New York, 2019. http://doi.org/10.1007/978-1-4939-9504-2_3.
[9] Johns, Douglas G., Louis-Charles Campeau, Puja Banka, An Bautmans, Tjerk Bueters, Elisabetta Bianchi, Danila Branca, et al. “Orally Bioavailable Macrocyclic Peptide That Inhibits Binding of PCSK9 to the Low Density Lipoprotein Receptor.” Circulation 148, no. 2 (July 11, 2023): 144–58. http://doi.org/10.1161/CIRCULATIONAHA.122.063372.
[10] Cm, Ballantyne, Banka P, Mendez G, et al. “Phase 2b Randomized Trial of the Oral PCSK9 Inhibitor MK-0616.” Journal of the American College of Cardiology 81, no. 16 (April 25, 2023). http://doi.org/10.1016/j.jacc.2023.02.018.
Related Services and Platforms




-

 In Vitro ADME ServicesLearn More
In Vitro ADME ServicesLearn More -

 Novel Drug Modalities DMPK Enabling PlatformsLearn More
Novel Drug Modalities DMPK Enabling PlatformsLearn More -

 Physicochemical Property StudyLearn More
Physicochemical Property StudyLearn More -

 Permeability and Transporter StudyLearn More
Permeability and Transporter StudyLearn More -

 Drug Distribution and Protein Binding StudiesLearn More
Drug Distribution and Protein Binding StudiesLearn More -

 Metabolic Stability StudyLearn More
Metabolic Stability StudyLearn More -

 Drug Interactions StudyLearn More
Drug Interactions StudyLearn More
Stay Connected
Keep up with the latest news and insights.











