In preclinical drug development, pharmacokinetic (PK) studies in rat models are essential [1]. They systematically evaluate a compound's ADME properties, generating critical PK parameters, and provide a critical foundation for clinical trials [2]. The reliability of this entire process hinges on one core step: the precision of blood sample collection. The industry's reliance on manual techniques has long been a bottleneck. To address these limitations, WuXi AppTec DMPK has successfully developed a fully proprietary Automated Blood Sampler (ABS). This instrument has passed rigorous internal validation and is already actively supporting research projects. This blog explores our proprietary ABS, detailing its methods and application in rat PK studies, and examining the transformative impact of automation in blood collection.
Traditional Practices in Blood Sample Collection
To minimize data variability and better adhere to the ethical 3R principles (Replacement, Reduction, and Refinement) [3], study designs often prioritize serial blood sampling from a single animal. Traditional techniques inclde the tail vein, retro-orbital plexus, and submandibular vein bleeding. However, each method has significant limitations [4].
High animal stress: elevated glucocorticoid (corticosterone) levels;
Poor sampling consistency: low standardization of technique;
High human error risk: human errors can occur.
Among those blood sampling methods, carotid artery cannulation presents significant strengths in serial sampling and minimizes animal stress, thus becoming a preferred method for the Automated Blood Sampler (ABS).
Brief introduction of the Automated Blood Sampler (ABS)
The Automated Blood Sampler (ABS) is comprised of four main systems: a blood recognition system, a sampling system, a software control system, and a specialized tubing system (See Figure 1). The instrument is designed to collect blood at precisely programmed time points, automatically recording the real time and volume for each sample.
Figure 1. Appearance of Automated Blood Sampler (ABS)
A key advantage is that animals can move freely during sampling, minimizing the stress induced by manual restraint and handling. This not only improves animal welfare but also produces more accurate PK data by reducing stress-induced physiological changes. Furthermore, the timing of blood collection is more precise, which can enhance the accuracy of PK parameters. The system’s hardware and software are protected by a Chinese invention patent and software copyright, respectively (Figure 2).

Figure 2. Chinese invention patent and computer software copyright certificate
The key features of the ABS include:
High precision sampling: syringe pump control allowing volume resolution to 0.1 μL.
Low‑adsorption tubing: minimizes analyte loss due to non-specific binding.
Intelligent data management: flexible protocol input, automatic timestamping, and secure data logging.
Integrated cold chain: immediate post‑collection refrigeration to preserve sample stability.
Rigorous Validation Studies of Automated Blood Sampler (ABS)
Validation 1: Assessing Cross-Sample Compound Carry-Over
Carry-over—where residue from a previous sample affects the next one—is a critical concern. It can be caused by compound adsorption to the tubing or incomplete system cleaning. To evaluate the ABS's performance, we tested three compounds representing different Biopharmaceutics Classification System (BCS) classes: metoprolol (BCS I), atenolol (BCS III), and furosemide (BCS IV).
The protocol had the ABS collect a sample for one compound, following an automatic system wash, then collect the next compound; the sequence was varied across three study runs to determine whether sampling order influenced results. Residual concentrations in subsequent samples were measured and carry‑over ratios calculated as (residual concentration/initial concentration) × 100%.
Table 1: Carry-over Validation Results of Automated Blood Sampler (ABS)
Analyte concentration (ng/mL) | |||||||||
Sample name | Study I | Study II | Study III | ||||||
Atenolol | Furosemide | Metoprolol | Metoprolol | Furosemide | Atenolol | Furosemide | Atenolol | Metoprolol | |
1st collection | 4976 | 4445 | 5414 | 5026 | 4686 | 4826 | 3929 | 3445 | 3975 |
2nd collection | 2.2 | 1.58 | -- | 14.2 | 3.58 | -- | 3.15 | 4.21 | -- |
Carry-over ratio | 0.04% | 0.03% | -- | 0.28% | 0.08% | -- | 0.08% | 0.12% | -- |
The carry-over ratio for all three compounds remained consistently below 0.5% across all study runs, comfortably meeting the acceptance criteria and ensuring sample integrity.
Validation 2: Automated Blood Sampler (ABS) vs. Manual Blood Sampling
This study compared ABS performance to traditional manual methods.
Animals: 6 male HW rats with carotid artery cannulation, divided into two groups (n=3).
Protocol: All animals received an IV dose of Atenolol (2 mg/kg). Blood was collected at 5, 15, 30 min, and 1, 2, 4, 6, 8, and 24 hours post-dose. One group was sampled using the ABS, the other manually.
Results: The plasma concentration-time curves from both groups (Figure 3) displayed nearly identical, classical biphasic profiles—consisting of a distribution phase (α, from 5min to 30min) and an elimination phase (β). The concentration values at each time point were very close between groups.
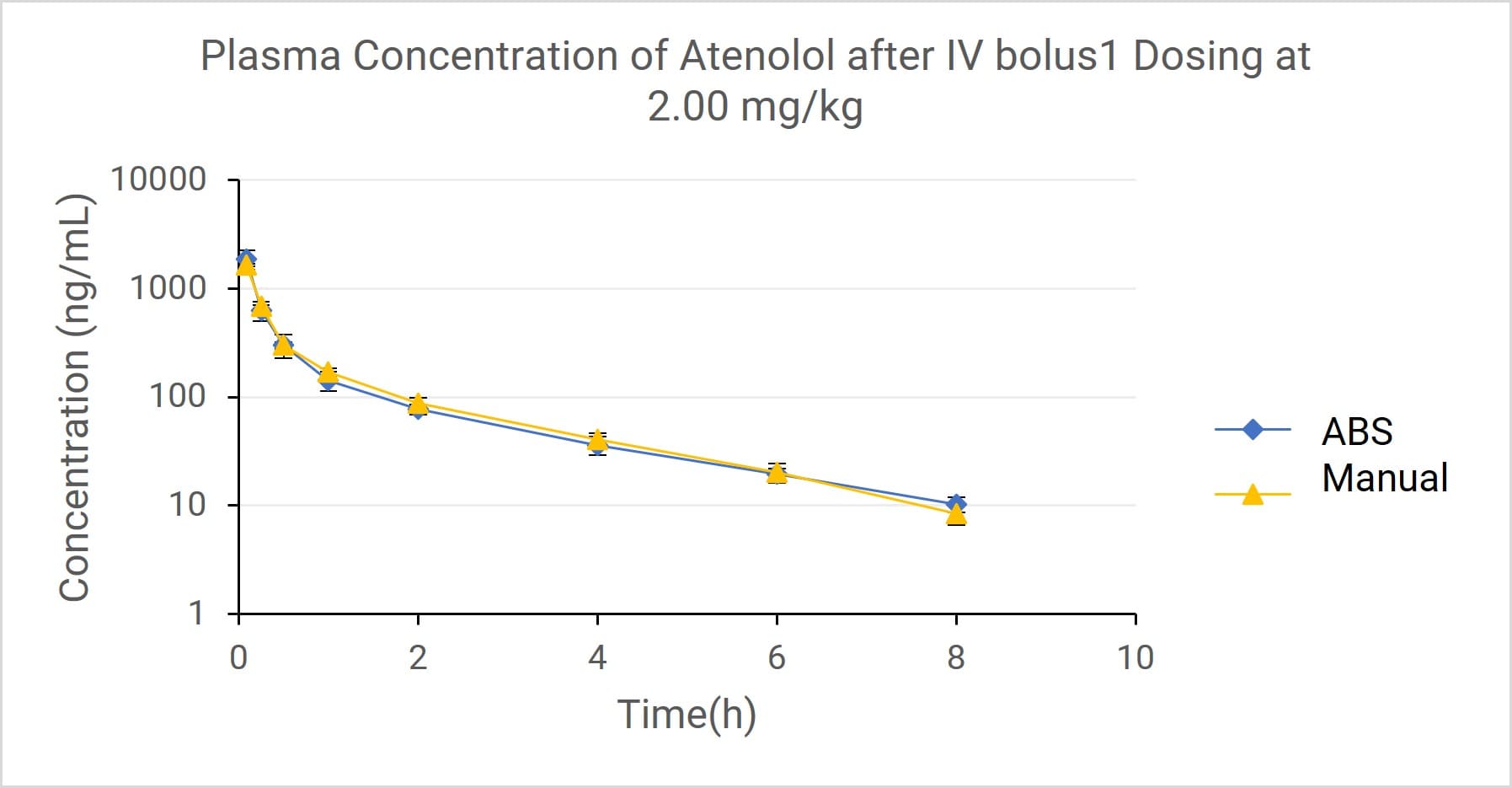
Figure 3. Plasma concentration of Atenolol after IV administration using Automated Blood Sampler (ABS) and manual blood sampling methods. Data points represent Mean ± SD (ng/mL).
Crucially, a statistical comparison of key PK parameters using a t-test showed no significant difference (P > 0.05) between the two sampling methods.
Table 2: PK Parameter Comparison (Mean ± SD)
PK Parameter | ABS Group (Mean ± SD) | Manual Group (Mean ± SD) | P-Value |
Vdss (L/kg) | 3.41 ± 0.65 | 3.12 ± 0.2 | 0.506 |
Cl (mL/min/kg) | 35.9 ± 5.64 | 35.4 ± 1.8 | 0.89 |
T1/2 (h) | 2.3 ± 0.49 | 1.8 ± 0.1 | 0.15 |
AUC0-last (ng·h/mL) | 910.3 ± 155 | 921.0 ± 42.1 | 0.91 |
These parameters were also consistent with values reported in scientific literature [5], further confirming the accuracy of the Automated Blood Sampler (ABS).
Conclusion
The validation data present a compelling case for the Automated Blood Sampler:
Minimal Carry-over: The consistently low carry-over ratio (<0.5%) confirms that the ABS maintains sample quality, preventing cross-contamination.
Data Consistency: The PK parameters and concentration curves derived from ABS sampling are statistically consistent with those obtained via manual sampling. The ABS delivers equally reliable data with higher standardization.
Final Perspectives
The integration of automation into blood sampling, as exemplified by the ABS, represents a significant advancement for preclinical research. Automated blood sampling significantly reduces animal stress. Studies have shown it produces markedly lower levels of the stress biomarker corticosterone compared to submandibular or tail vein methods [6] [7].
Beyond animal welfare, the ABS enhances data quality through standardized collection procedures, ensuring precise sampling times and generating more reliable, reproducible results. WuXi AppTec DMPK ABS is a validated tool already generating robust data in rat PK studies, empowering scientists to advance drug candidates with greater confidence and efficiency.
Authors: Yunxi Chen, Jiajia Li, Zhiqiang Hu, Honglei Ma, Li Wang, Furong Jiao, Cheng Tang
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Lin J H, Lu A Y H. Role of pharmacokinetics and metabolism in drug discovery and development[J]. Pharmacological reviews, 1997, 49(4): 403-449.
[2] Di L, Kerns E H. Drug-like properties: concepts, structure design and methods from ADME to toxicity optimization[M]. Academic press, 2015.
[3] Russell W M S, Burch R L, Hume C W. The principles of humane experimental technique[M]. London: Methuen, 1959.
[4] Gjendal K, Kiersgaard M K, Abelson K, et al. Comparison of sublingual, facial and retro-bulbar blood sampling in mice in relation to animal welfare and blood quality[J]. Journal of Pharmacological and Toxicological Methods, 2020, 103: 106680.
[5] Mehvar R, Gross M E, Kreamer R N. Pharmacokinetics of atenolol enantiomers in humans and rats[J]. Journal of pharmaceutical sciences, 1990, 79(10): 881-885.
[6] Holmberg A, Pelletier R, DiLab A B. Automated blood sampling and the 3Rs[J]. National Center for the Replacement, Refinement and reduction of Animals in Research, 2009, 16.
[7] Teilmann A C, Kalliokoski O, Sørensen D B, et al. Manual versus automated blood sampling: impact of repeated blood sampling on stress parameters and behavior in male NMRI mice[J]. Laboratory animals, 2014, 48(4): 278-291.
Related Services and Platforms




Stay Connected
Keep up with the latest news and insights.











