In recent years, with rapid advances in monoclonal antibody and Fc engineering, the neonatal Fc receptor (FcRn), a long-neglected component of the immune system, has emerged as a focal point in drug development. By acting as the key "gatekeeper" that regulates the half-life of IgG antibodies, FcRn has become a compelling therapeutic target, and FcRn inhibitors have shown substantial potential, particularly for autoimmune diseases. This article outlines the mechanism of action of FcRn, reviews progress in FcRn inhibitors, and highlights critical considerations for bioanalytical assessment.
Mechanism of Action: FcRn Therapeutic Targeting for Autoimmune Diseases
FcRn inhibitors are designed to treat autoimmune diseases, which are generally categorized as organ-specific or systemic.
Organ-Specific
Driven by autoantibodies that directly attack antigens within a given organ, leading to local inflammation and tissue damage. Examples include Myasthenia Gravis (MG), Immune Thrombocytopenia (ITP), Goodpasture’s syndrome, and Autoimmune Hemolytic Anemia (AHA).
Systemic
Caused by autoantibodies that bind free molecules, cell surface antigens, or nucleoprotein antigens to form pathogenic immune complexes (ICs). Examples include Rheumatoid Arthritis (RA), Systemic Lupus Erythematosus (SLE), and Cryoglobulinemic Vasculitis (CV).
In organ-specific autoimmune diseases, IgG autoantibodies target antigens within specific tissues. FcRn-mediated recycling prolongs the half-life and tissue residence of these pathogenic IgGs, thereby exacerbating immune damage. FcRn inhibitors accelerate their clearance and reduce antibody-mediated attacks on the affected organ. For example, in Pemphigus Vulgaris (PV), blocking FcRn with efgartigimod protects keratinocyte monolayers from anti-Dsg3-IgG-mediated loss of adhesion, indicating that FcRn inhibitors act not only systemically but also confer local tissue protection [4].
For systemic autoimmune diseases, pathology is driven by elevated levels of pathogenic IgG autoantibodies, which activate complement and/or form deposits in tissues, causing inflammatory damage. Traditional treatments, such as broad-spectrum immunosuppressants or anti-CD20 therapies, often have a slow onset of action, high relapse rates, and notable adverse effects. FcRn inhibitors provide a rapid-acting, mechanism-based alternative: by competitively inhibiting Fc fragment-FcRn binding, they promote the lysosomal degradation of IgG - including pathogenic autoantibodies, thereby lowering serum IgG levels. Notably, this strategy does not affect other immunoglobulin classes like IgM or IgA, nor does it impact vaccine-induced immune memory [5].
Current Landscape of FcRn Inhibitors
Based on the mechanisms described above, current FcRn inhibitors are developed around two principal strategies:
High-affinity competition: Deploy molecules with high affinity to FcRn to competitively block IgG recycling and promote IgG degradation [6].
pH-independent binding: Design molecules that bind FcRn at both neutral and acidic pH to enable comprehensive intracellular and extracellular blockade. Modalities include peptides, anti-FcRn monoclonal antibodies (mAbs), FcRn-specific Fab-fusion proteins, Fc-engineered IgG, and affibody proteins [7].
These preclinical findings (Figure 1) have laid a solid foundation for advancing FcRn inhibition in IgG-mediated autoimmune diseases.
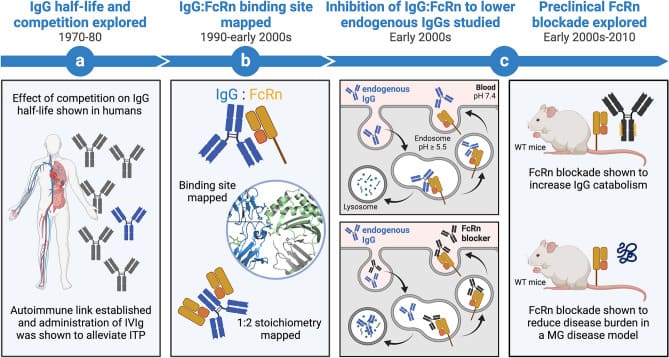
Figure 1. Evolution of functional research on neonatal Fc receptor (FcRn) in disease [7]
Currently, five FcRn inhibitors have entered clinical development, with several other protein-based molecules in preclinical stages expected to enter clinics between 2025 and 2026. Table 1 summarizes the characteristics and data of these candidates [7].
To date, two FcRn antagonists have been approved for marketing:
Efgartigimod (ARGX-113, Brand name: VYVGART): The first FcRn antagonist approved by the FDA (2021). An engineered Fc fragment indicated for adults with generalized Myasthenia Gravis (gMG) who are anti-AChR antibody-positive. Available in both intravenous and subcutaneous formulations, and under investigation for Immune Thrombocytopenia (ITP), Chronic Inflammatory Demyelinating Polyneuropathy (CIDP), and Pemphigus Vulgaris (PV).
Rozanolixizumab (Brand name: Rystiggo): The first subcutaneous FcRn antagonist, approved in 2023 for adults with gMG who are anti-AChR- or anti-MuSK-antibody positive.
These approvals clinically validate the core principle that lowering circulating IgG levels confers therapeutic benefit, providing strong evidence for the continued development of FcRn inhibitors.
Table 1. Approved and investigational neonatal Fc receptor antagonist
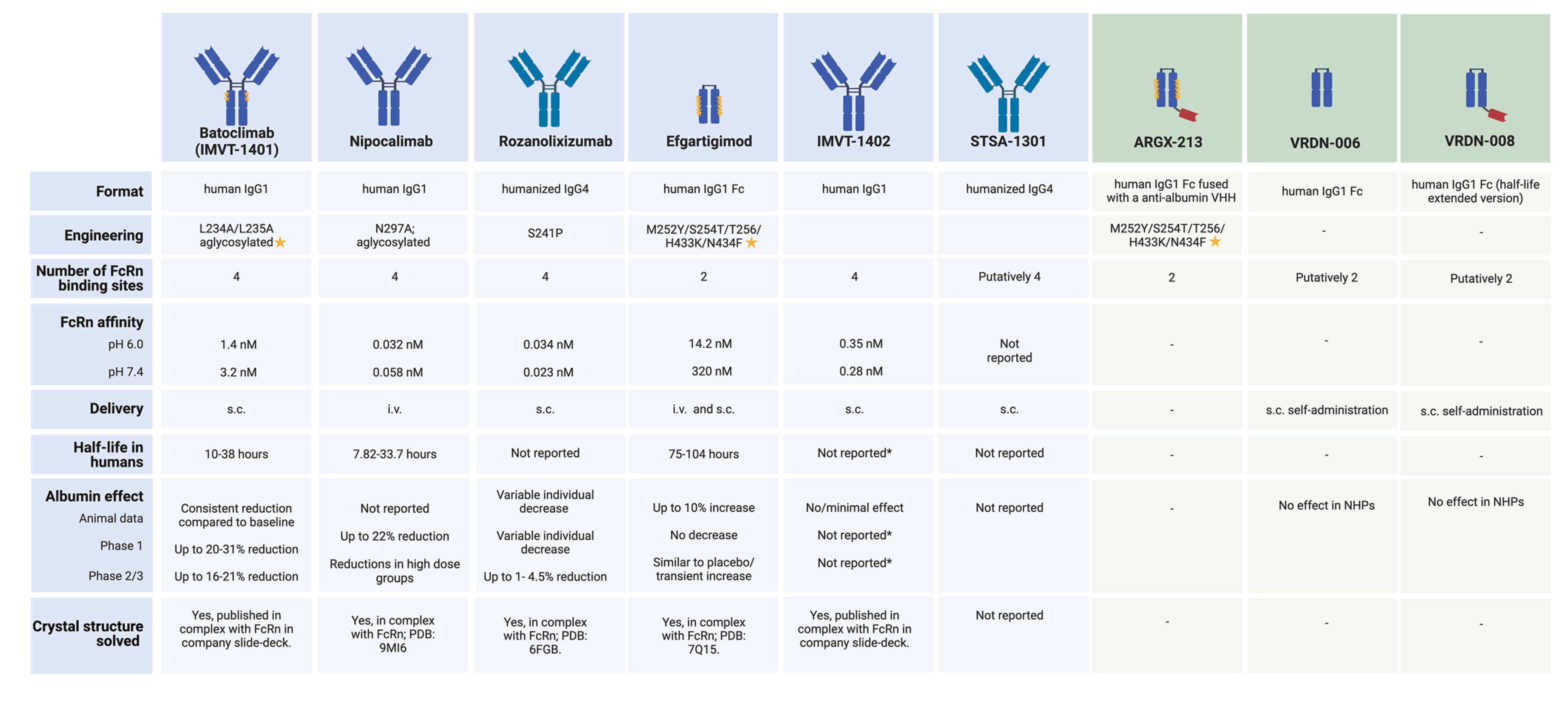
Blue: Active clinical development and/or use phase; Green: Preclinical phase
Key Bioanalytical Strategies and Metrics for FcRn Inhibitors
Selecting appropriate pharmacokinetic (PK) and pharmacodynamic (PD) markers is essential for evaluating efficacy and safety in the development of FcRn-targeted drugs. Equally important is choosing the right bioanalytical platform. Ligand Binding Assays (LBA) serve as the gold standard for large molecule bioanalysis and, for FcRn therapeutics, support comprehensive assessment across PK, PD, and immunogenicity.
Optimizing Pharmacokinetic (PK) Analysis
Pharmacokinetics primarily involves monitoring drug concentration changes in the blood to derive parameters such as half-life and clearance, thereby assessing in-vivo exposure and the appropriateness of the dosing regimen.
Most FcRn antibody drugs are Human IgG or fragment structures (e.g., Fc fragments, Fab fusion proteins). PK assays typically target the antibody backbone. While a generic assay format using anti-Fc, anti-heavy/light chains, or anti-Fab conserved-region reagents is routine for rodent studies (mice, rats), significant challenges arise in non-human primates (NHP). Due to the high homology with human IgG, these reagents may cross-react with endogenous NHP IgG, causing signal interference. This can manifest as false positives (non-specific binding amplification) or false negatives (competitive occupation of binding sites).
Therefore, PK methods must possess high sensitivity and specificity to distinguish the drug from endogenous IgG. To improve specificity and accuracy, the following strategies are commonly employed (Figure 2):
Use anti-idiotype antibodies that recognize unique epitopes.
Optimizing acid dissociation conditions to release the drug from endogenous complexes without compromising assay performance.
Introducing competitive clearance steps to minimize interference from endogenous IgG or Fc-containing species.
Additionally, rapid dynamic changes in a patient's total IgG levels during treatment can significantly impact background signals, sample dilution ratios, and the linear range of calibration curves. Long-term monitoring requires careful attention to sampling time points (e.g., distinguishing between the rapid IgG decline phase post-dosing and the recovery phase), dilution linearity verification, and individual variations in immune status.
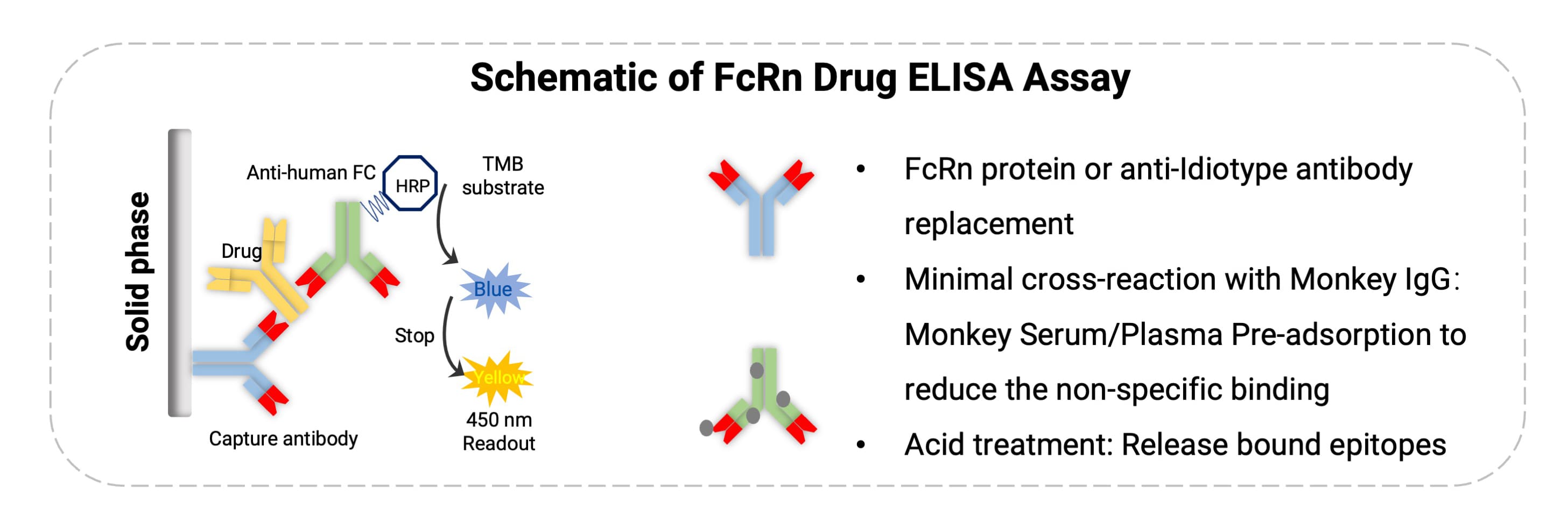
Figure 2. Analytical strategies for FcRn antibody drugs
A 2015 study evaluated the pharmacokinetics of efgartigimod in healthy volunteers. In single ascending dose (SAD) studies, efgartigimod showed a rapid peak concentration (2 hours) after intravenous administration, dose-proportional AUC at medium-to-high doses, effective distribution (Vz≈15-21 L), and a half-life of approximately 3.5–4.5 days with minimal urinary excretion. Multiple dosing showed no significant accumulation, providing a reliable PK basis for dosing strategies aimed at rapid, controlled IgG reduction.
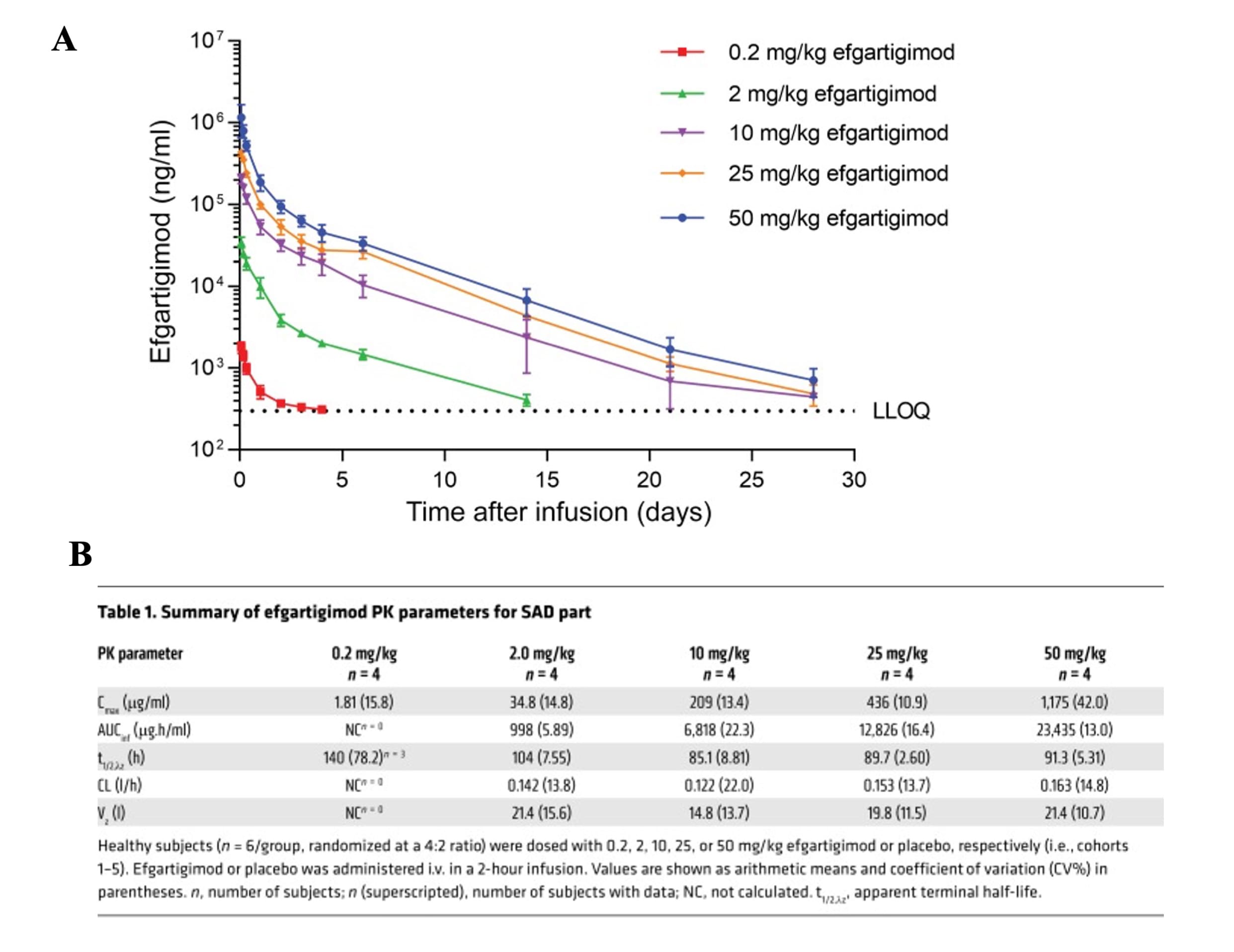
Figure 3. Plasma concentration and PK parameters of Efgartigimod in human trials [8]
Pharmacodynamic (PD) Markers and Efficacy Assessment
For FcRn inhibitors, PD analysis generally focuses on: the magnitude of total IgG decline, dynamic changes in specific autoantibodies (e.g., AChR antibodies, platelet-associated antibodies), changes in IgG subclass ratios (IgG1-4), and plasma albumin levels (to assess the risk of hypoalbuminemia caused by FcRn inhibition) [7].
In human trials for efgartigimod, compared to the placebo group, single doses of 2 to 50 mg/kg resulted in a rapid, sustained, and dose-dependent decrease in IgG levels (including IgG1, IgG2, IgG3, and IgG4). Interestingly, the reduction across different IgG subtypes was very similar over time, although IgG4 reduction was slightly less pronounced. This may suggest that the production rate or FcRn interaction profile of IgG4 differs from other subtypes. Efgartigimod did not significantly alter IgA, IgD, IgE, or IgM levels, as the endogenous levels of these immunoglobulins do not depend on FcRn-mediated recycling [8].
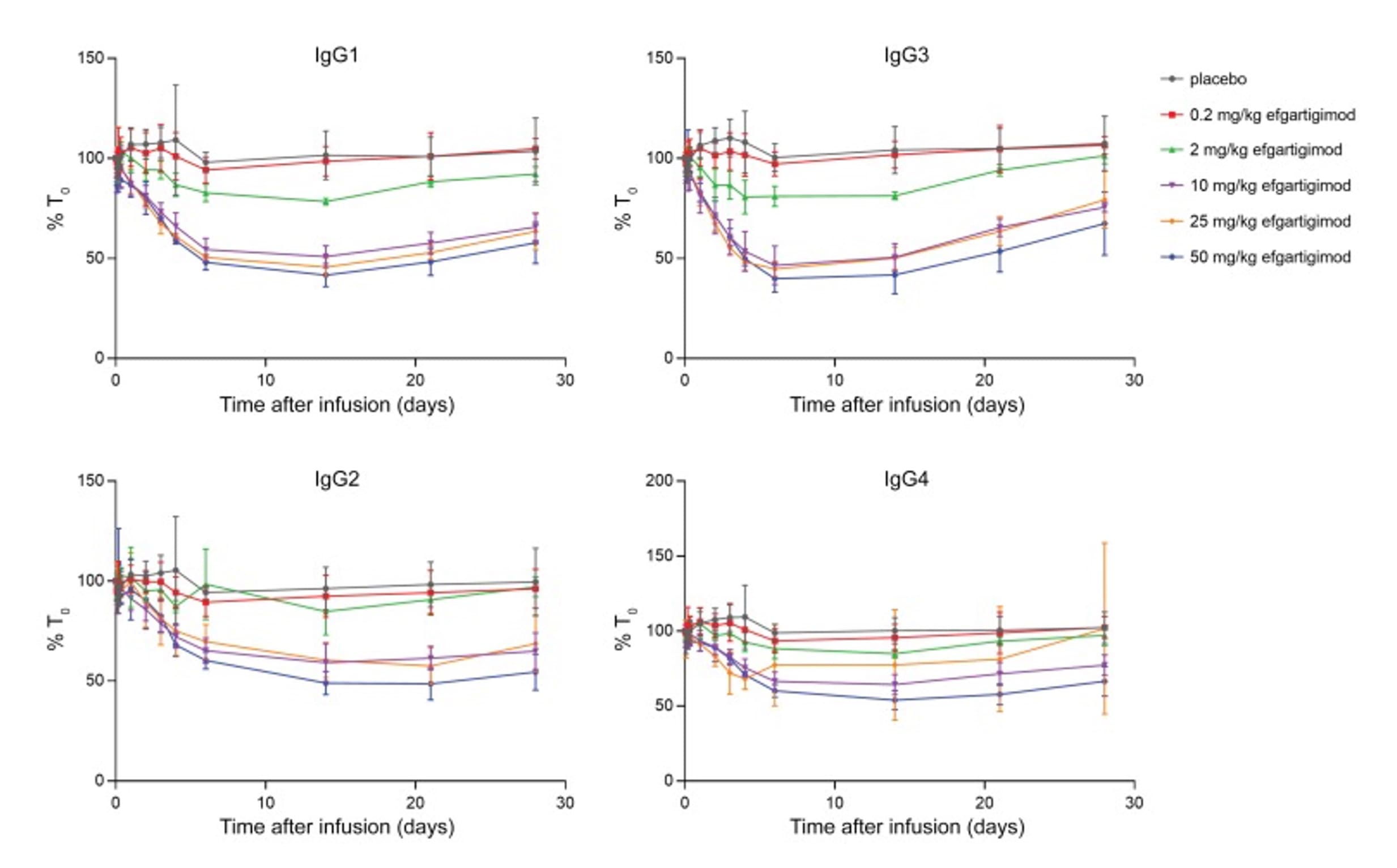
Figure 4. Changes in serum IgG subclass levels over time in the first-in-human study of Efgartigimod [8]
Since the FcRn binding characteristics of Cynomolgus monkey IgG and human IgG are very similar, and efgartigimod exhibits increased affinity for both species' FcRn in a comparable manner, Cynomolgus monkeys are considered a relevant species for exploring efgartigimod's pharmacodynamics.
For endogenous monkey IgG analysis, in addition to routine clinical biochemistry, ELISA methods can achieve higher sensitivity and specificity (see Figure 5). WuXi AppTec DMPK has established and validated a Monkey IgG ELISA method. Since most FcRn-targeted drugs are based on a Human IgG backbone, which shares high homology with Monkey IgG, many commercial detection antibodies recognize both species.
When quantifying Monkey IgG, the drug itself (human IgG architecture) can potentially interfere with the assay. Based on our internal data, within the 50–5000 ng/mL linear range for Monkey IgG, the addition of 10–250 ng/mL of Human IgG (to simulate drug presence) produced no significant interference. This tolerance level covers the drug Cmax at doses up to 500 mpk, ensuring the method remains applicable even under high drug exposure.
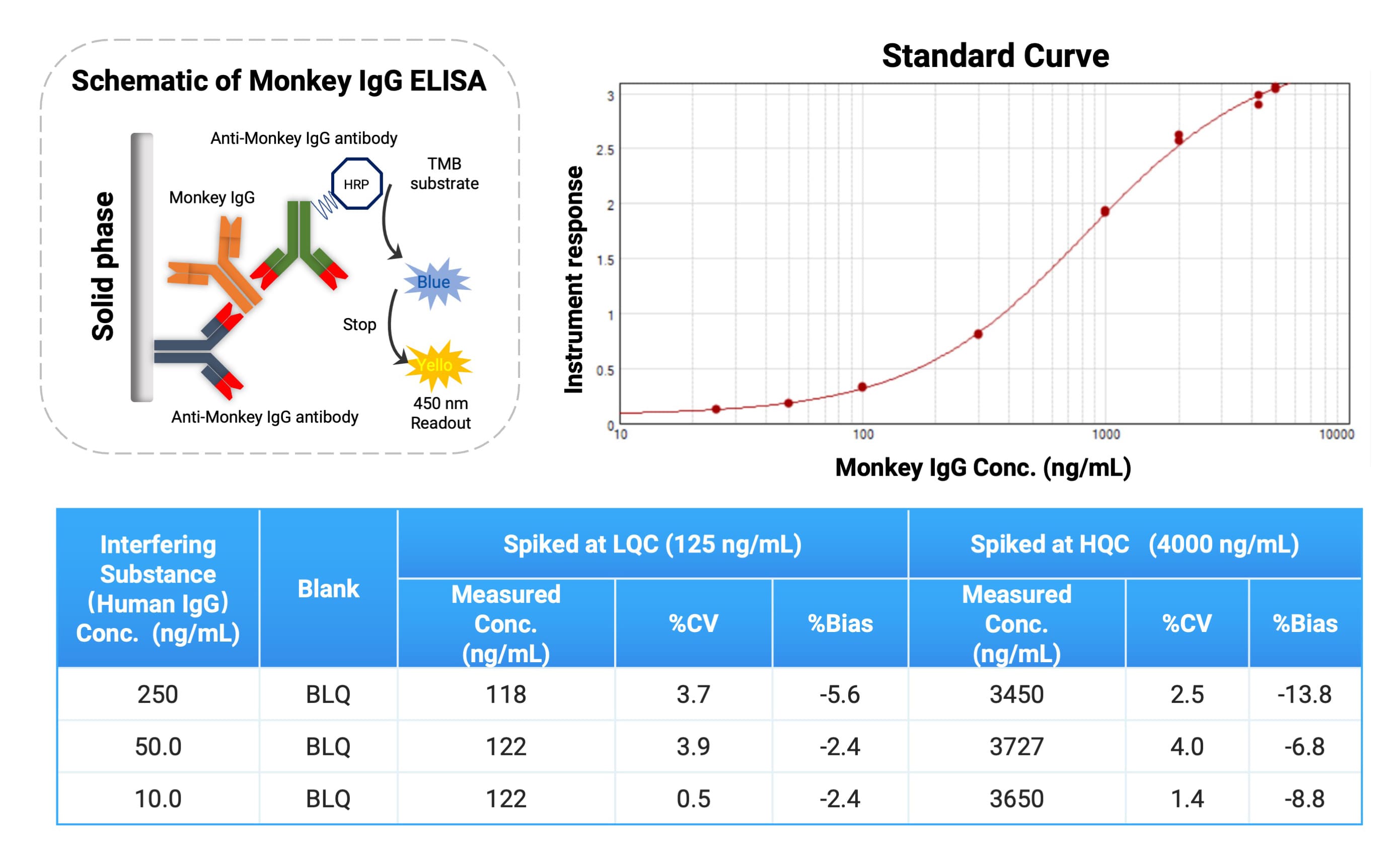
Figure 5. Analytical strategy for endogenous monkey IgG
Notably, endogenous Monkey IgG levels may vary substantially between individuals. We established baseline levels across multiple subjects for reference. In PK/PD experiments for such drugs, pre-screening animals by baseline Monkey IgG is often advisable to ensure that subjects with appropriate levels are selected for in vivo studies (Figure 6).
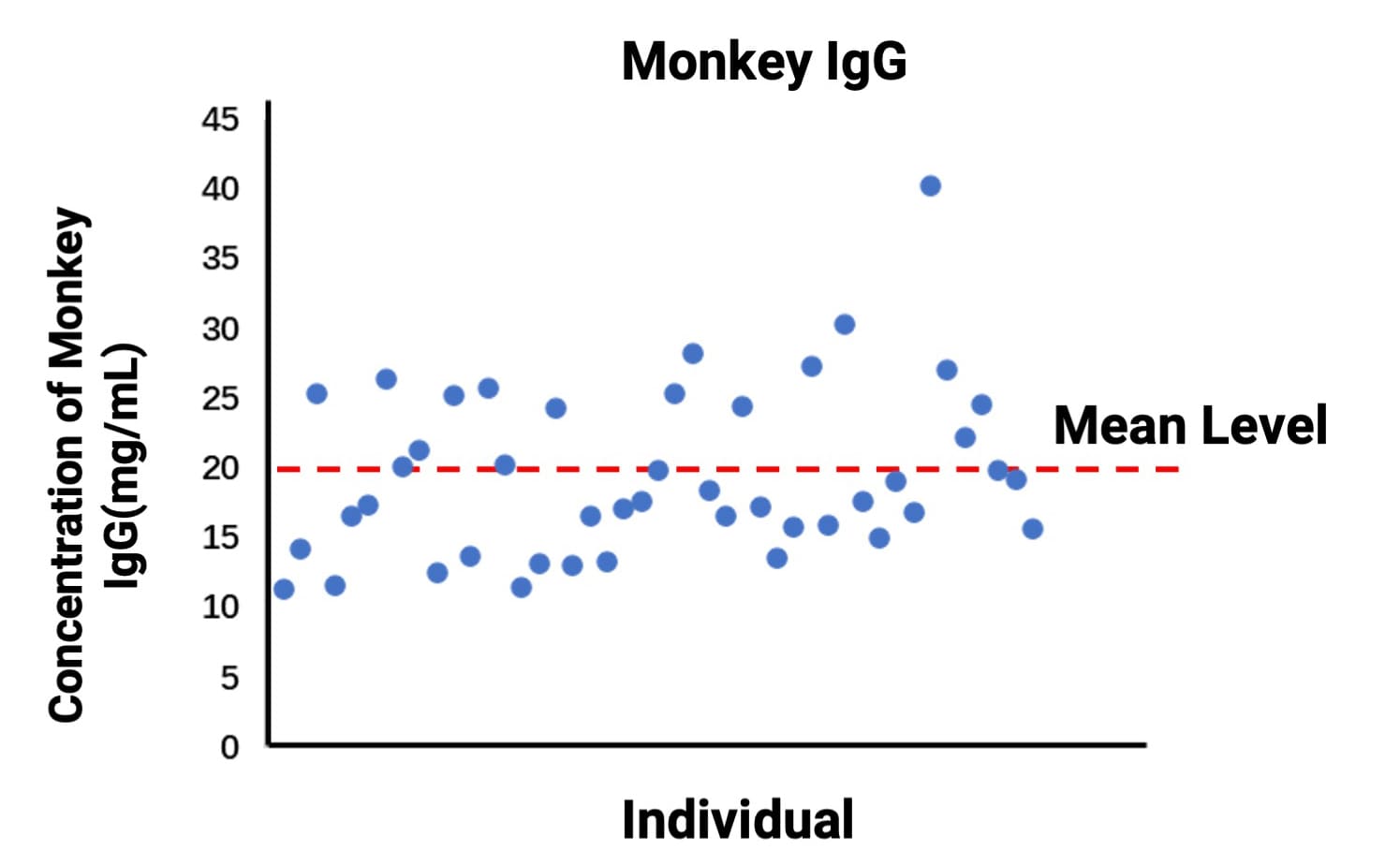
Figure 6. Baseline levels of endogenous monkey IgG
Special Considerations for Immunogenicity Assessment of FcRn Inhibitors
As large molecule biologics, FcRn inhibitors may induce anti-drug antibodies (ADA). The existence of ADA can affect pharmacokinetics and pharmacodynamics, leading to reduced drug exposure, diminished efficacy, or increased risk of immune-related adverse events.
Uniquely, because FcRn inhibitors substantially lower total IgG levels in the body, the reduction in background IgG may alter the baseline signals and dynamic range in ADA assays, thereby impacting detection sensitivity and cut-point settings. Therefore, ADA assay design and validation must explicitly account for this factor to ensure accurate and reliable results.
Implementing a Comprehensive Bioanalytical Framework
Bioanalysis of FcRn inhibitors requires balancing sensitivity, specificity, and method robustness. Only through optimized assay design and rigorous validation can drug exposure and immune response be assessed accurately, providing reliable data to support clinical development. This framework applies to approved FcRn inhibitors and serves as a practical reference for pipeline candidates.
Concluding Remarks
Targeting the neonatal Fc receptor (FcRn) provides an innovative approach for treating autoimmune diseases. Mechanistically, FcRn inhibition accelerates the clearance of pathogenic IgG, shortens its residence time, and reduces its opportunity to mediate tissue injury. Across both organ-specific and systemic autoimmune diseases, FcRn physiologically extends IgG half-life; interrupting this pathway provides a new option when conventional therapies fail.
Compared with traditional broad-spectrum immunosuppressive therapies, FcRn inhibitors offer a more selective mechanism, faster clinical response, and potentially more favorable safety profile, making them a key focus of development in autoimmune diseases.
WuXi AppTec DMPK possesses a mature and comprehensive ligand binding assay (LBA) platform with experience spanning early screening to IND submission. We provide end-to-end capabilities in PK/PD sample analysis, biomarker monitoring, and immunogenicity assessment. Having supported dozens of FcRn-targeted projects, we bring extensive, modality-specific bioanalytical expertise.
For the development of FcRn-targeted biologics (antibodies, fusion proteins, etc.), we provide:
In vivo PK characterization of candidate drugs.
PD assessment of drug-induced IgG reduction.
Immunogenicity risk monitoring.
Furthermore, our LBA platform integrates synergistically with LC-MS/MS, qPCR, and flow cytometry platforms to deliver high-quality analytical support and robust data for FcRn-targeted drug development.
Authors: Duo Zhang, Xue Zhang, Wenrui Ren, Huan Yan, Miaomiao Song, Lili Xing
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Saxena A, Wu D. Advances in Therapeutic Fc Engineering - Modulation of IgG-Associated Effector Functions and Serum Half-life. Front Immunol. 2016;7:580.
[2] Patel DD, Bussel JB. Neonatal Fc receptor in human immunity: Function and role in therapeutic intervention. J Allergy Clin Immunol. 2020;146(3):467-78.
[3] Pyzik M, Kozicky LK, Gandhi AK, Blumberg RS. The therapeutic age of the neonatal Fc receptor. Nat Rev Immunol. 2023;23(7):415-32.
[4] Zakrzewicz A, Vanderheyden K, Galaly Y, Feldhoff S, Sips M, Brinkhaus M, Tikkanen R. Binding to the neonatal Fc receptor enhances the pathogenicity of anti-desmoglein-3 antibodies in keratinocytes. Front Immunol. 2024;15:1473637.
[5] Howard JF, Jr., Bril V, Vu T, Karam C, Peric S, De Bleecker JL, et al. Long-term safety, tolerability, and efficacy of efgartigimod (ADAPT+): interim results from a phase 3 open-label extension study in participants with generalized myasthenia gravis. Front Neurol. 2023;14:1284444.
[6] Zhu LN, Hou HM, Wang S, Zhang S, Wang GG, Guo ZY, Wu J. FcRn inhibitors: a novel option for the treatment of myasthenia gravis. Neural Regen Res. 2023;18(8):1637-44.
[7] Gjolberg TT, Mester S, Calamera G, Telstad JS, Sandlie I, Andersen JT. Targeting the Neonatal Fc Receptor in Autoimmune Diseases: Pipeline and Progress. BioDrugs. 2025;39(3):373-409.
[8] Ulrichts P, Guglietta A, Dreier T, van Bragt T, Hanssens V, Hofman E, et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest. 2018;128(10):4372-86.
Related Services and Platforms




-

 DMPK BioanalysisLearn More
DMPK BioanalysisLearn More -

 Novel Drug Modalities DMPK Enabling PlatformsLearn More
Novel Drug Modalities DMPK Enabling PlatformsLearn More -

 Novel Drug Modalities BioanalysisLearn More
Novel Drug Modalities BioanalysisLearn More -

 Small Molecules BioanalysisLearn More
Small Molecules BioanalysisLearn More -

 Bioanalytical Instrument PlatformLearn More
Bioanalytical Instrument PlatformLearn More -

 PROTAC DMPK ServicesLearn More
PROTAC DMPK ServicesLearn More -

 ADC DMPK ServicesLearn More
ADC DMPK ServicesLearn More -

 Oligo DMPK ServicesLearn More
Oligo DMPK ServicesLearn More -

 PDC DMPK ServicesLearn More
PDC DMPK ServicesLearn More -

 Peptide DMPK ServicesLearn More
Peptide DMPK ServicesLearn More -

 mRNA DMPK ServicesLearn More
mRNA DMPK ServicesLearn More -

 Covalent Drugs DMPK ServicesLearn More
Covalent Drugs DMPK ServicesLearn More
Stay Connected
Keep up with the latest news and insights.