Drug metabolism studies are integral throughout the entire drug development process. Metabolite identification research helps elucidate the biotransformation pathways of compounds, clarify whether active or reactive metabolites are produced, and obtain information on whether high-proportion or unique metabolites are formed in humans. The derivatization method holds unique advantages in both metabolite identification and quantitative analysis. This article primarily summarizes the definition of derivatization, its application in metabolite identification by LC-HRMS (liquid chromatography–high resolution mass spectrometry), common reaction types, and case studies of chemical derivatization methods used in metabolite identification.
What is Derivatization
Chemical derivatization involves performing a derivatization reaction between a specifically designed chemical derivatization reagent and the target compound containing specific active functional groups in a sample. This reaction structurally modifies a particular functional group of the target compound. While ensuring its basic skeleton structure remains unchanged, its physicochemical properties are altered, thereby achieving the goal of changing the chromatographic and mass spectrometric behavior of the target compound (Figure 1). In essence, it utilizes chemical reactions to convert substances that are difficult to analyze into structurally similar substances that are easier to analyze, facilitating separation, detection, and identification [1].
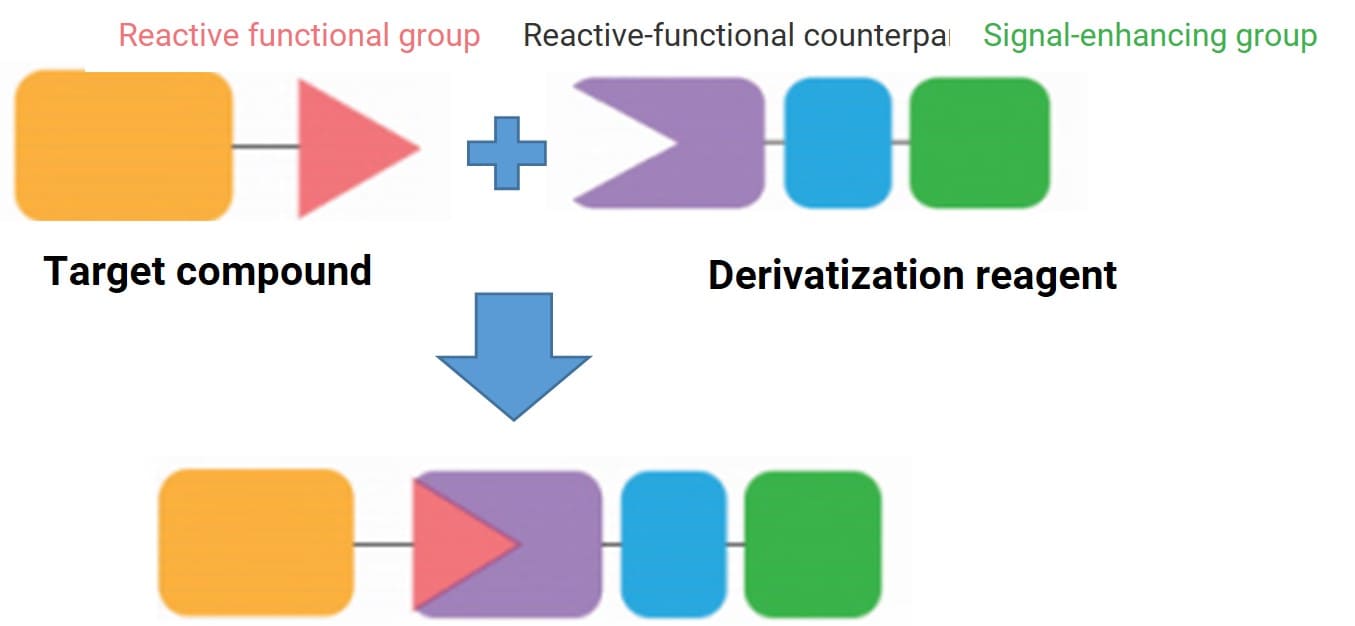
Figure 1. Principle of the derivatization method [1]
Derivatization reactions serve many purposes [2,3], mainly including:
Altering volatility and/or thermal stability, making compounds more suitable for LC-MS analysis.
Optimizing chromatographic behavior: 1) Improving chromatographic retention; 2) Improving peak shape, reducing or eliminating peak tailing or fronting; 3) Improving separation efficiency.
Enhancing detectability: 1) Generating more abundant ions; 2) Reducing ion interference; 3) Improving sensitivity and reproducibility.
Why Use Derivatization in LC-HRMS
HRMS (high-resolution mass spectrometers) are crucial analytical tools for drug metabolite analysis and identification. Despite ongoing iterations and updates in detection instruments and auxiliary tools, numerous challenges persist in the analysis and identification of metabolites. For example: compounds with small molecular weight and high polarity are often poorly retained in reversed-phase chromatography and are susceptible to ion suppression and matrix interference during detection; metabolites of compounds containing structures like purines, pyrimidines, nucleotides, and amino acids are easily interfered with by endogenous substances in the matrix, making chromatographic separation difficult; compounds such as steroids, terpenoids, liposomes, which generally lack nitrogen or other heteroatoms or contain few heteroatoms, typically have no UV response and poor MS response, making them difficult to detect and thus identify; and for metabolites present in high abundance, with potential activity or toxicity, precise structural information cannot be obtained solely from mass spectrometry data, among many other challenges.
Typically, these challenges can be partially addressed by methods such as optimizing chromatographic conditions, isotope tracing, synthesizing possible metabolite reference standards for comparison, biosynthetic, followed by NMR identification, H/D exchange, and chemical derivatization. Each of the aforementioned methods has its own advantages and limitations. The appropriate method can be selected based on a comprehensive judgment of the actual application scenario (Table 1). Derivatization holds the strengths of broad scope, flexible options, and reliable results, and is widely used in metabolite identification and quantitative analysis.
Table 1. Methods for assisting in metabolite identification research by LC-HRMS and their advantages and limitations
Method | Advantages | Limitations |
Optimizing Chromatographic Conditions | Fast, simple, readily available | Narrow scope, only solves problems for common types of compounds |
Isotope Tracing | Accurate, intuitive, comprehensive | Requires synthesis of labeled compounds and conducting experiments in specific facilities |
Comparison with Synthesized Reference Standards | Simple, accurate (preferred when readily available) | Reference standards can be difficult to obtain, expensive, and time-consuming |
Biosynthetic Followed by NMR Identification | Non-destructive, recoverable, and storable long-term, allowing multiple analyses on the same sample. | Metabolites generally exist at low concentrations in complex matrices, requiring separation, purification, and enrichment to a certain amount; time-consuming |
H/D Exchange | Targets labile hydrogens, convenient operation | Requires deuterated reagents, a narrow application scope, and only solves a small subset of problems |
Chemical Derivatization | Broad application scope, many reaction options, relatively convenient operation, and reliable results | Destructive, not suitable for complex matrices or extensively metabolized products, requires separation/enrichment beforehand |
Selection of Chemical Derivatization Reagents
The selection of chemical derivatization reagents depends on the properties of the target compound and the research objectives. Generally, they should possess the following characteristics:
The derivatization reagent undergoes a stable derivatization reaction with the compound and is unaffected by complex matrices.
The derivatization reagent can react rapidly with the target compound under mild conditions, generating minimal non-target by-products, or the generated derivatization by-products do not affect instrument detection.
The derivatization reagent must not interfere with the analysis of the analyte and should be easily removable.
The derivatization reagent should be readily available or synthesizable.
The derivatization reagent should cause minimal instrument damage and be low-toxicity or non-toxic.
Types of Chemical Derivatization Reactions
When using derivatization techniques to assist in the qualitative or quantitative analysis of target compounds in biological matrices, most chemical derivatization reactions can be categorized into three types based on the introduced group: Silylation, Acylation, and Alkylation [2,3].
Silylation Reaction
Silylation refers to the introduction of a silyl group into the target compound, typically by replacing the labile hydrogen of the target compound with an alkyl-silyl group, especially in reactions with compounds containing -OH, generating silyl compounds. This reduces the polarity of the original compound, enhances its volatility and stability, and increases its retention in chromatography (Figure 2). Common functional groups containing labile hydrogens include alcohol hydroxyl, phenolic hydroxyl, carboxylic acid, amino, thiol, and amide groups (the ease of silylation reaction generally decreases in that order).
Reaction Conditions:
For non-volatile or compounds stable at 200-300°C.
Solvents are typically hexane, benzene, pyridine, tetrahydrofuran, DMSO, acetonitrile, etc.
Silylation reagents are prone to moisture absorption and deactivation, thus requiring dehydration of samples and reagents during use; the reaction system must be free of water.
Common silylation reagents include BSTFA (N,O-Bis(trimethylsilyl)trifluoroacetamide), TMCS (Trimethylchlorosilane), MSTFA (N-Methyl-N-(trimethylsilyl)trifluoroacetamide), and HMDS (Hexamethyldisilazane).

Figure 2. Silylation derivatization reaction
Acylation Reaction
The principle of acylation is the reaction of alcohols or amines in the target compound with acid anhydrides, generating esters (more stable and often higher sensitivity derivatives) for detection (Figure 3). Acylation reactions can reduce the polarity of hydroxyl, amino, etc., groups, minimize chromatographic peak tailing, enhance the volatility of the original compound; improve the stability of certain easily oxidized compounds (e.g., catecholamines), and introduce acyl groups containing halogen ions to increase sensitivity in Electron Capture Detectors (ECD). Common alcohol or amine functional groups include alcohol hydroxyl, phenolic hydroxyl, and amino groups. Common acylation derivatization reactions include acyl anhydride derivatization and acyl imidazole derivatization.
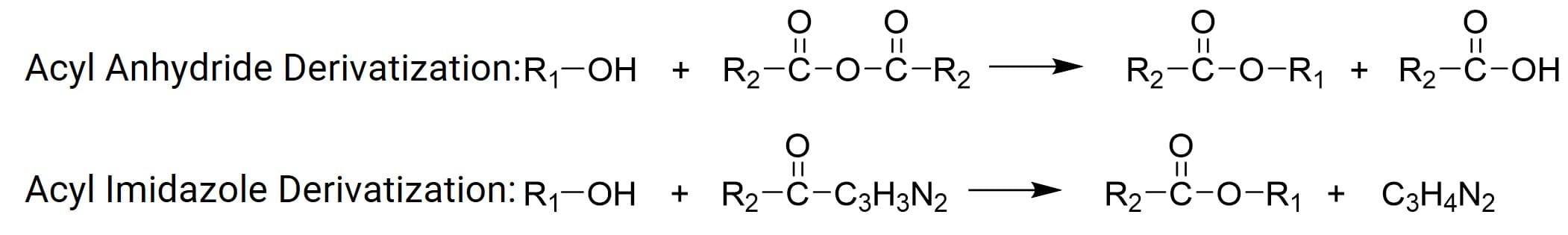
Figure 3. Acylation chemical derivatization
Alkylation Reaction
The principle of alkylation is the replacement of labile hydrogens in the target compound with alkyl groups, thereby reducing the polarity of the original compound and increasing its retention in reversed-phase chromatography. Common functional groups containing labile hydrogens include alcohol hydroxyl, amino, thiol, amide, carboxylic acid, and phenolic hydroxyl. Taking methylation as an example, common reactions include (Figure 4):
Alkyl iodide reactions: e.g., methylation using iodomethane under reflux in dry acetone containing potassium carbonate.
Methylation using TMPAH (specifically for barbiturates).
Methylation reactions using diazomethane as the reagent.
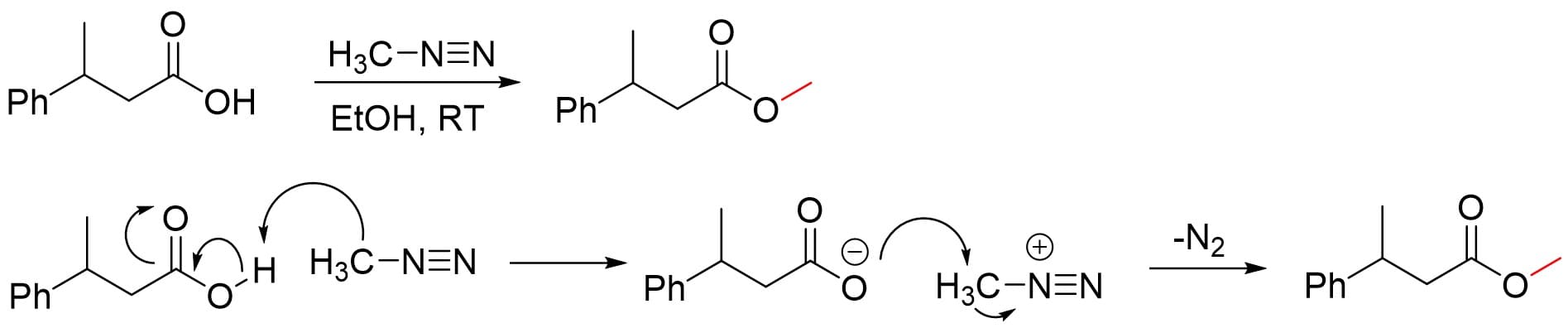
Figure 4. Alkylation chemical derivatization
Derivatization in Metabolite Identification and Quantification Analysis: Case Studies
Case Study 1: Derivatization Assists in Glucuronic Acid Conjugation Site Identification
For the marketed compound Alvimopan (P), glucuronide conjugates of the parent drug were detected in in vitro incubation samples (liver microsomes + UDPGA). This structure contains multiple potential glucuronidation sites, such as phenolic hydroxyl, carboxylic acid, and nitrogen. Based solely on MS data, without key fragment information, the specific conjugation site cannot be determined. In such cases, derivatization methods demonstrate their unique advantages. Acetylation reactions can differentiate between glucuronide conjugates at the carboxylic acid site and non-carboxylic acid sites (Figure 5), and trimethylsilylation reactions can differentiate between glucuronide conjugates at the phenolic hydroxyl site and non-phenolic hydroxyl sites (Figure 6) [4].
Based on the trimethylsilylation derivatization reaction (where TMS (trimethylsilyl) binds to the phenolic hydroxyl or the hydroxyl groups of glucuronic acid), an increase in molecular weight by 3×72 or 4×72 Da can indicate glucuronide conjugation at the phenolic hydroxyl (3×72) or at the carboxylic acid and nitrogen sites (4×72), respectively. Based on the acetylation derivatization reaction (where ethyl binds to the carboxylic acid), an increase in molecular weight by 1×28 or 2×28 Da can indicate glucuronide conjugation at the carboxylic acid (1×28) or at the phenolic hydroxyl and nitrogen sites (2×28), respectively. Through these two types of derivatization reactions, it can be determined whether Alvimopan's glucuronide is conjugated to the phenolic hydroxyl, carboxylic acid, or nitrogen.
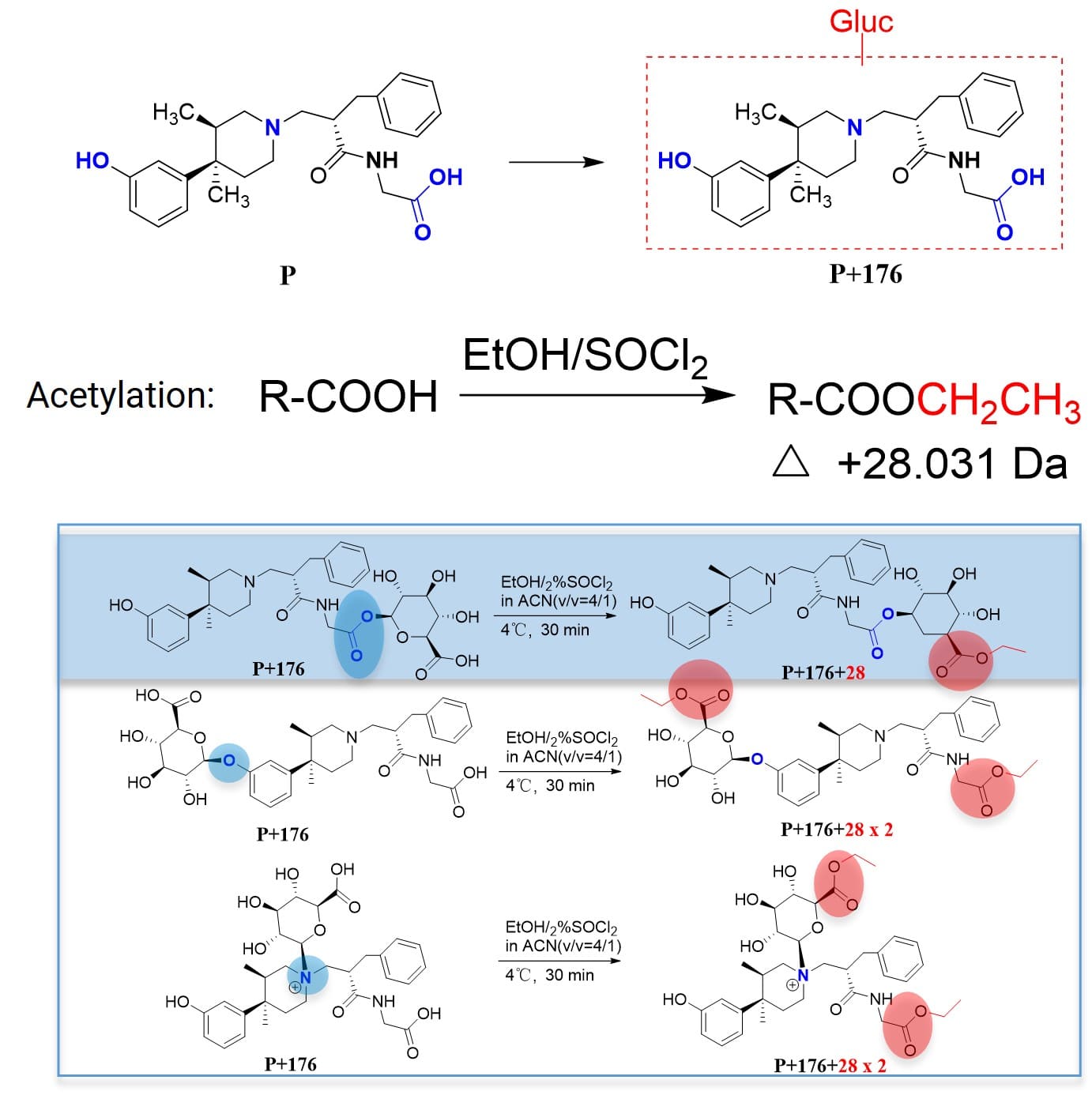
Figure 5. Determining glucuronic acid conjugation site via acetylation reaction (determining whether at the carboxylic acid site) [4]
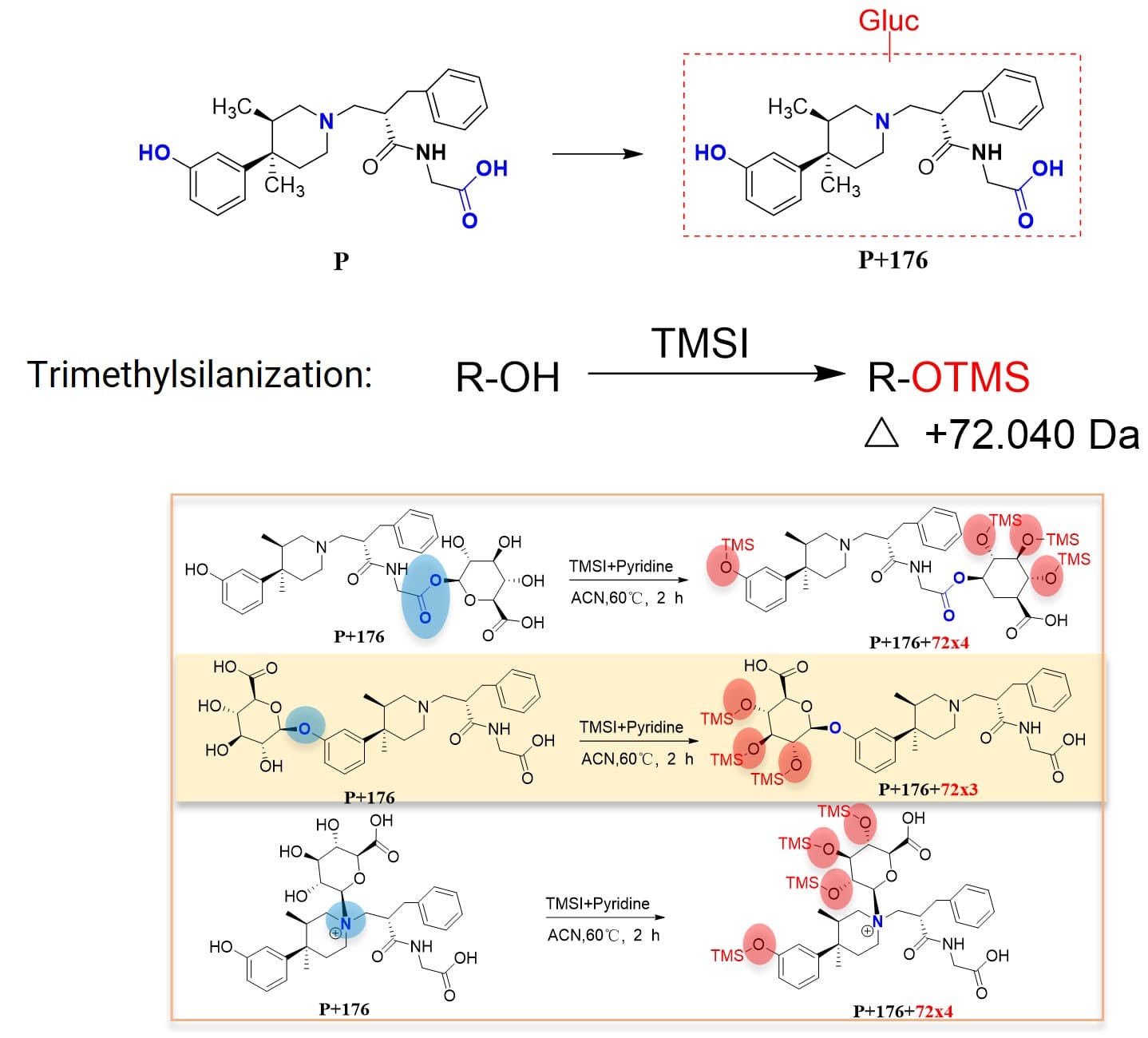
Figure 6. Determining glucuronic acid conjugation site via trimethylsilylation reaction (determining whether at phenolic hydroxyl site) [4]
Case Study 2: Derivatization Assists in the Identification of Metabolites, same as Endogenous materials
A nucleotide analog small molecule was labeled with 14C on its nucleotide moiety to investigate its metabolic pathway in rats. After oral administration to rats, plasma, urine, and feces samples were collected. The urine samples were centrifuged and analyzed via liquid chromatography coupled with online radiometric detection (LC-RAM), yielding a radio-metabolite profile containing only one metabolite (M1). Mass spectrometric detection of this metabolite M1 revealed that it co-eluted with and had a similar MS response to the endogenous substance allantoin (m/z 157.0367), making it impossible to confirm whether M1 was allantoin (Figure 7). Changing the HPLC method still failed to separate M1 from endogenous allantoin. Therefore, the urine sample was further subjected to an acetylation derivatization reaction. After the reaction, M1 was converted into four radioactive derivatives D1~D4. Analysis of their radio-metabolite profiles and high-resolution mass spectra identified D1~D4 as multi-acetylated products of allantoin. Integrating this information confirmed that the radioactive metabolite M1 was allantoin (Figure 8).
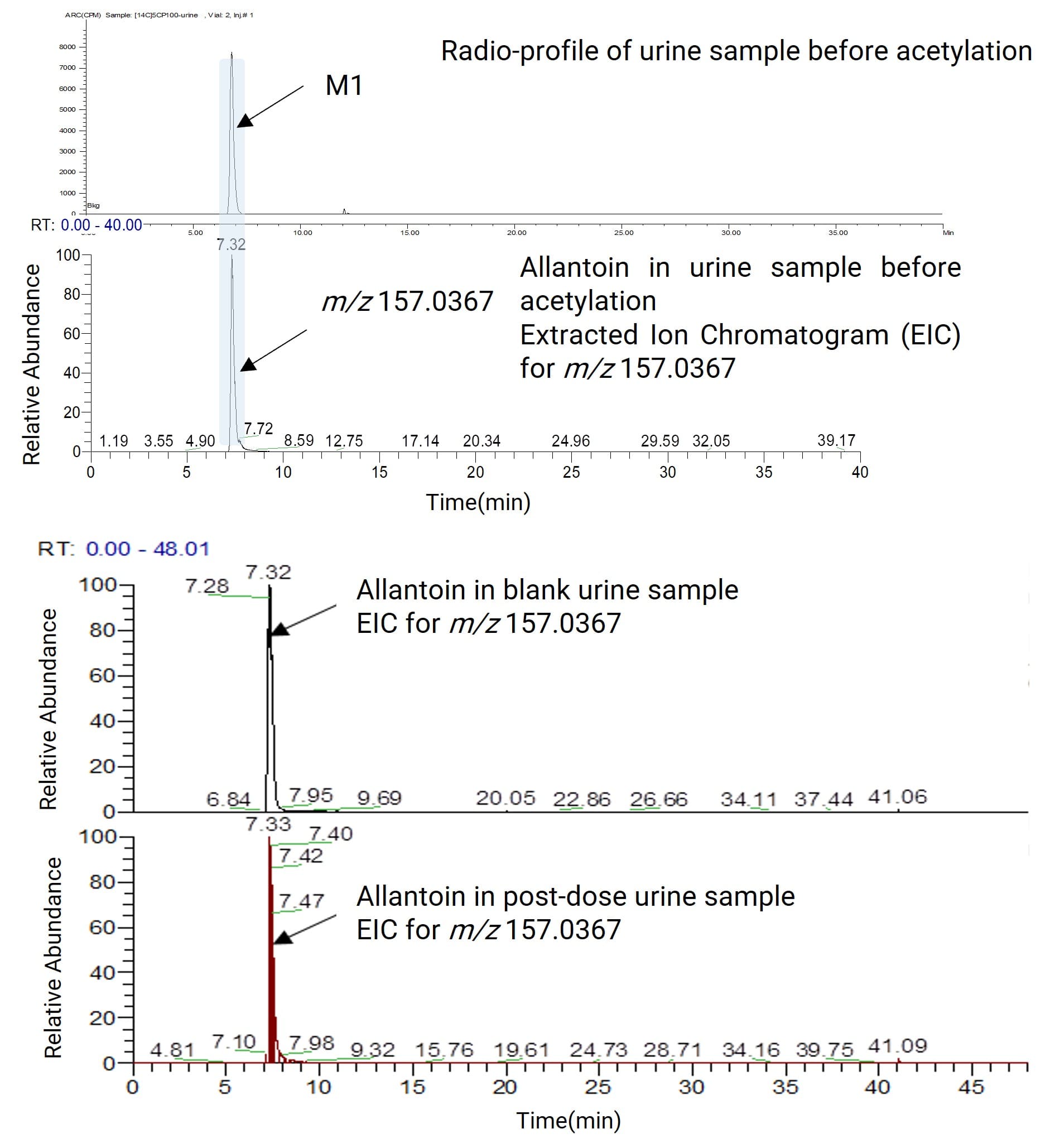
Figure 7. Radiometric profile of metabolite M1 in a urine sample and its mass spectrometric comparison with allantoin
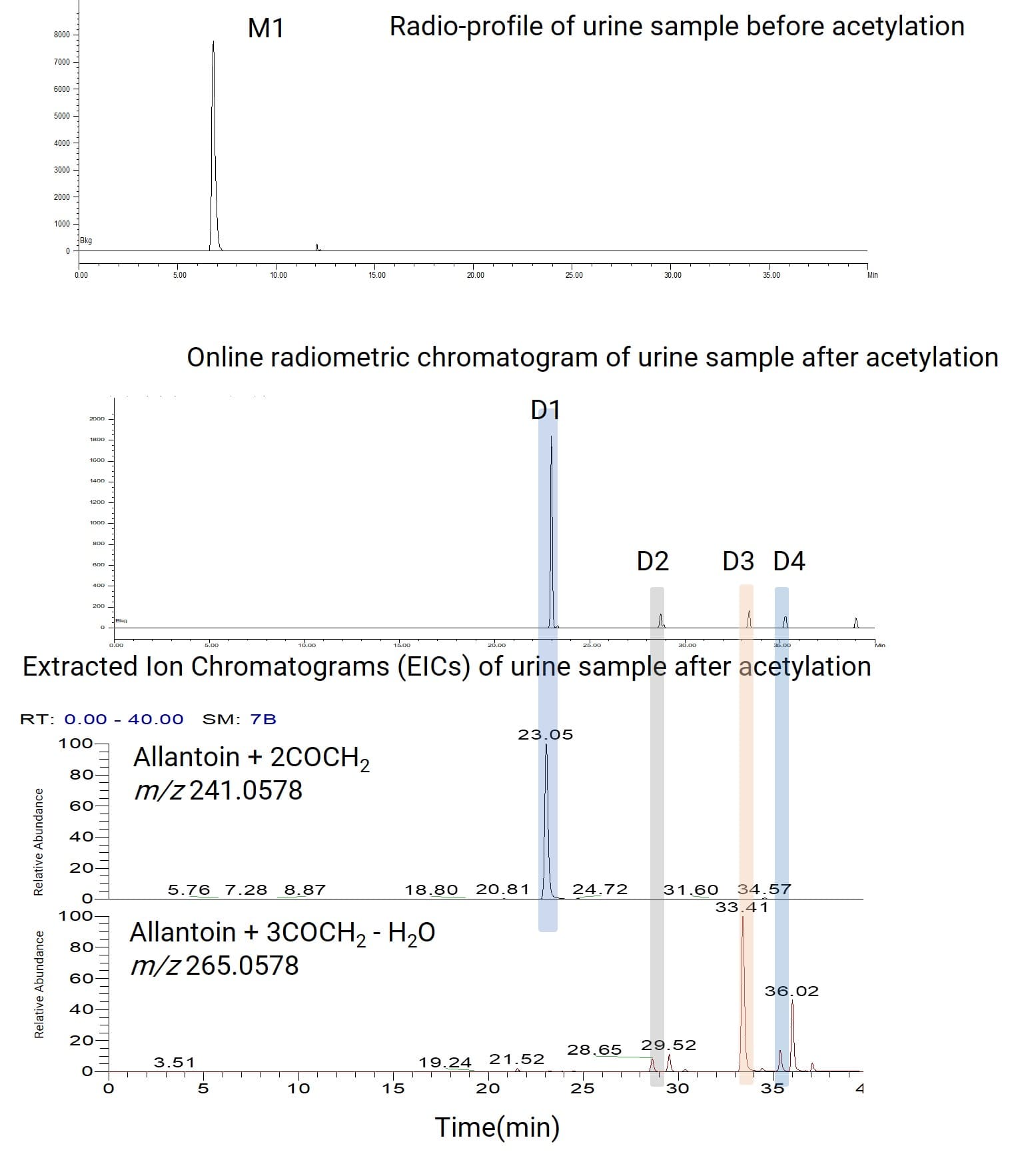
Figure 8. Generation of radioactive derivatives D1-D4 from M1 in the urine sample via acetylation
Case Study 3: Derivatization Enhances Target Compound Sensitivity and Lowers Detection Limits
Beyond aiding in the qualitative study of metabolites, chemical derivatization's contribution is even more prominent in quantitative analysis. Ethaselen ([M+H]+: 425) contains selenium in its molecule but lacks labile hydrogens, resulting in poor ionization efficiency, low response in mass spectrometry, and severe matrix interference. Quantitative LC-MS/MS analysis of Ethaselen in plasma samples post-dosing was exceedingly difficult. Targeting its structure, a derivatization reaction using dimercaptoethanol reagent was employed. By optimizing the derivatization reaction conditions in plasma samples, Ethaselen was converted into a selenium-containing derivative ([M+H]+: 581). Compared to Ethaselen, the mass spectrometric response of this derivative was enhanced by 10-fold, improving the signal-to-noise ratio, eliminating matrix interference, and lowering the lower limit of quantification (LLOQ) to 50 ng/mL. This facilitated the quantitative analysis of this compound in plasma samples [5] (Figure 9).
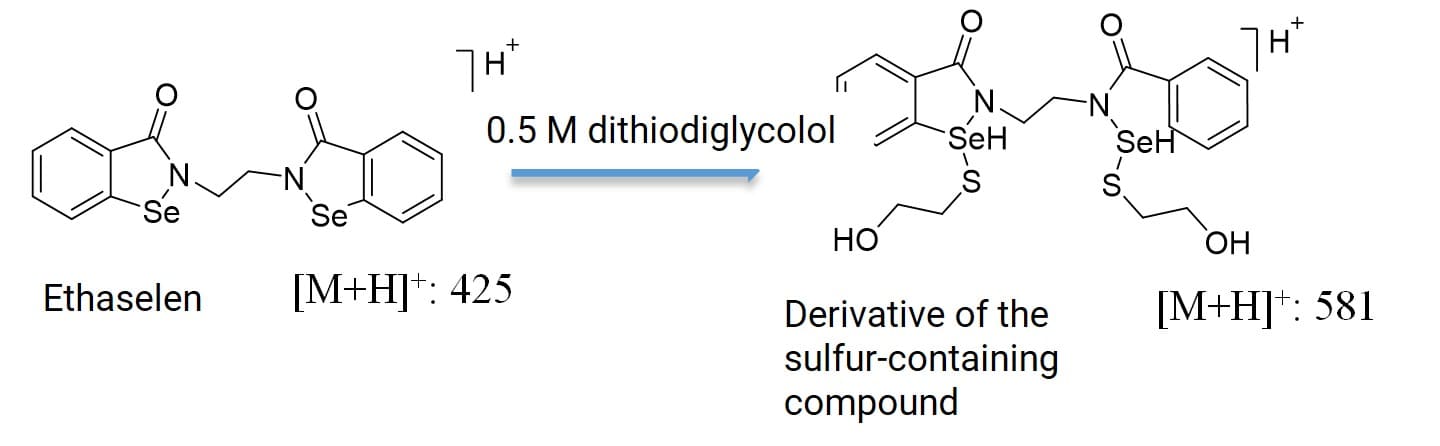
Figure 9. Derivatization reaction of an Ethaselen [5]
Concluding Remarks
Addressing the numerous analytical challenges in metabolite identification research, we employ high-resolution mass spectrometry analysis, leveraging the advantages of derivatization reactions, literature research, case studies, and existing experimental experience. This targeted approach helps solve specific analytical difficulties in metabolite identification, aiding in obtaining more reliable metabolite identification results. Beyond the silylation, acylation, and alkylation derivatization methods detailed in this article, there are many other chemical derivatization approaches. As illustrated in Case Study 3 above, specific derivatization methods can be developed for compounds containing special elements. We have summarized the reaction mechanisms and selection strategies of representative derivatization reagents to guide and inspire future metabolism research. Furthermore, derivatization reactions find numerous applications in peptide and protein analysis, environmental analysis, pharmaceutical analysis, food safety assessment, and MS imaging, among others.
WuXi AppTec DMPK possesses extensive experience in metabolite identification, with thousands of metabolite identification studies conducted annually. Over the past decade, we have empowered clients to complete over a thousand regulatory submissions. We provide global clients with comprehensive pharmacokinetic services spanning early-stage screening, preclinical development, and clinical stages, supporting clients in accelerating their drug development pipelines.
Authors: Xiaoju Zhou, Huan Li, Lian Guo, Lingling Zhang, Weiqun Cao
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Yuqi Cao, Yingjie Lu, Yinlong Guo. Research Advances in Chemical Derivatization-Mass Spectrometric Analysis Methods for Alcohol Metabolites in Biological Samples[J]. Journal of Chinese Mass Spectrometry Society, 2021, 42(05): 727-743.
[2] Michael J. Telepchak, Thomas F. August, Glynn Chaney, Forensic and Clinical Applications of Solid Phase Extraction, Chapter 12, Chemical Derivatization, 297~309.
[3] Bao-Ling Qi, Ping Liu, Qiu-Yi Wang, Wen-Jing Cai, Bi-Feng Yuan, Yu-Qi Feng, Derivatization for liquid chromatography-mass spectrometry, Trends in Analytical Chemistry (2014), http://dx.doi.org/doi:10.1016/j.trac.2014.03.013.
[4] Yukuang Guo, Abhi Shah, Eugene Oh, Swapan K Chowdhury, Xiaochun Zhu, Determination of Acyl-, O-, and N- Glucuronide Using Chemical Derivatization Coupled with Liquid Chromatography-High-Resolution Mass Spectrometry, Drug Metab Dispos, 2022 May;50(5):716-724
[5] Hai-Yan Zhou, Gui-Fang Dou, Zhi-Yun Meng, Ya-Qing Lou, Guo-Liang Zhang, High performance liquid chromatographic determination of 1,2-[bis(1,2-benzisoselenazolone-3(2H)-ketone)]-ethane (BBSKE), a novel organoselenium compound, in dog plasma using pre-column derivatization and its application in pharmacokinetic study, Journal of Chromatography B, Volume 852, Issues 1–2, 2007, Pages 617-624
Related Services and Platforms




-

 MetID (Metabolite Profiling and Identification)Learn More
MetID (Metabolite Profiling and Identification)Learn More -

 DMPK BioanalysisLearn More
DMPK BioanalysisLearn More -

 In Vitro MetID (Metabolite Profiling and Identification)Learn More
In Vitro MetID (Metabolite Profiling and Identification)Learn More -

 In Vivo MetID (Metabolite Profiling and Identification)Learn More
In Vivo MetID (Metabolite Profiling and Identification)Learn More -

 Metabolite Biosynthesis and Structural CharacterizationLearn More
Metabolite Biosynthesis and Structural CharacterizationLearn More -

 Metabolites in Safety Testing (MIST)Learn More
Metabolites in Safety Testing (MIST)Learn More -

 Novel Drug Modalities BioanalysisLearn More
Novel Drug Modalities BioanalysisLearn More -

 Small Molecules BioanalysisLearn More
Small Molecules BioanalysisLearn More -

 Bioanalytical Instrument PlatformLearn More
Bioanalytical Instrument PlatformLearn More
Stay Connected
Keep up with the latest news and insights.