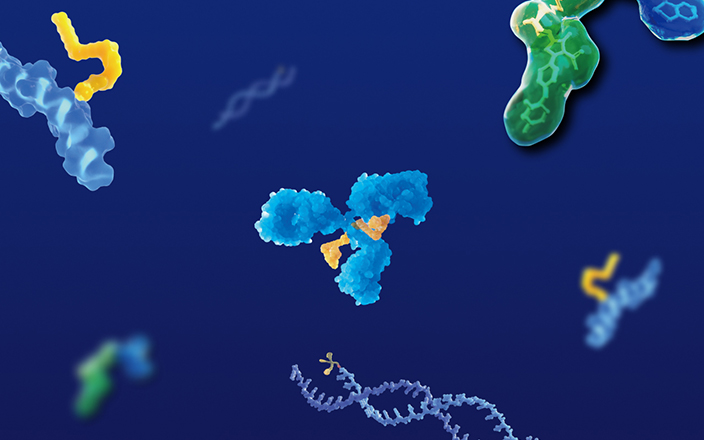
Antibody-Drug Conjugates (ADCs) are a class of drugs that conjugate monoclonal antibodies and payloads through a linker. Its basic structure is shown in Figure 11. ADCs combine highly- targeted monoclonal antibodies with strong cytotoxicity payloads for precision targeting. As of the end of 2022, 14 ADCs have been approved by FDA and more than 100 ADC drugs are undergoing clinical trials2.

Figure 1. Schematic diagram of the molecular structure of ADC
ADCs have complex structures and diversified forms. The guidelines for ADC pharmacokinetics (PK) studies have yet to be formulated by FDA and ICH organizations around the world. This makes ADC PK studies extremely challenging and fraught with uncertainty. However, ignoring ADC PK studies, or substituting them, only with toxicological data, could result in unanticipated complications for your program.
In PK studies, bioanalysis plays an indispensable role in presenting concrete results of each testing stage. From complete ADC drug-to-antibody ratio (DAR) analysis to PK study methods of each ADC component, this article will present strategies for ADC bioanalysis based on the experience of our team of DMPK experts.
1. The Overall Strategy for ADC Bioanalysis
ADCs have the characteristics of both small molecule and macromolecular therapeutics. Therefore, the ADC bioanalysis methods must consider these unique characteristics. Specifically, the ADC bioanalysis strategies can be divided into three following aspects that cover the discovery, pre-clinical development, and clinical development stages:
(1) ADC DAR characterization in animal plasma/serum: ADCs with different Drug-Antibody Ratios (DARs) may have completely different PK and toxicological characteristics and could even be considered two different drugs. Inaccurate pre-clinical study results or unpredictable toxicity may occur when ADCs are roughly studied as a mixture. Therefore, accurate ADC DAR characterization is necessary.
We have developed a new method to characterize the molecular structure of ADCs in plasma/serum to determine analytes with different DARs in circulation. This method provides data in in vitro/in vivo processes of ADCs (e.g. whether antibodies still carry most covalently bound drugs over time).
(2) Quantitative analysis of ADC-related components: According to its structure and in vivo transformation characteristics, ADC analytes undergoing PK analysis are usually divided into total antibody, ADC, conjugated payload, unconjugated payload, payload-related metabolites, and anti-therapeutic antibody (ATA).
Combined with traditional LBA (ligand binding assays), small molecule LC-MS/MS assays, affinity capture LC-MS/MS, affinity capture LC-HRMS, and other new analytical methods, a feasible quantitative analysis method was developed to conduct quantitative analysis of ADC components in animal plasma/serum (see Table 1).
Among these approaches, the LC-MS method offers the advantages of having a short time cycle and good versatility. The method has also demonstrated utility in macromolecular quantitative analysis. This article will focus on the LC-MS method and elaborate on it with examples.
Analytes | Definition | Analytical Methods | Special Abilities |
Total antibody | DAR≥0 | ELISA&LC-MS | LC–HRMS intact protein analysis & DAR analysis |
ADC | DAR≥1 | ELISA&LC-MS | |
Conjugated payload | Small molecule drugs conjugated to antibodies | LC-MS |
|
Unconjugated payload | Released small molecule drug | LC-MS |
|
Payload related metabolites | Payload-related components with medicinal properties generated after metabolism | LC-MS |
|
ATA | Anti-therapeutic antibody | ELISA |
|
Table 1. Definitions of ADC components and methods of bioanalysis
(3) Anti-therapeutic antibody (ATA) investigation of ADCs: Appropriate methods should be developed to determine the ATA of ADCs. At present, this part is frequently completed with a platform of ligand binding assays (LBA). WuXi AppTec DMPK has a dedicated LBA platform.
2. ADC DAR Characterization
2.1 What is DAR?
DAR (drug-to-antibody ratio, which represents the number of payloads conjugated to one antibody) is an important parameter used to describe the physiochemical properties of ADCs. It indicates the payload carried by a single ADC molecule, which in turn relates to ADC safety and effectiveness.
Most ADCs currently approved use heterogeneous conjugation technology (e.g., amine-based lysine conjugation or thiol-based cysteine conjugation). Since each antibody has multiple Lys/Cys loci, and the conjugation process is random, the number and location of the payload formed by conjugation are uncertain, and the ADC eventually obtained is often a mixture of multiple components with different DARs. For example, T-DM1 (as shown in Figure 2), has a DAR that ranges from 0 to 7, with an average DAR of approximately 3.5.
DAR will affect the ADC stability in in vivo circulation. Typically, an ideal DAR is 3-4. An overly high DAR will increase drug clearance, decrease half-life, and may lead to drug aggregation as well as increased toxicity.
 Figure 2. T-DM1 DAR characterization results. T-DM1 is a mixture of ADCs with different DARs. With the LC-HRMS platform, the characterization of ADCs with different DARs can be realized
Figure 2. T-DM1 DAR characterization results. T-DM1 is a mixture of ADCs with different DARs. With the LC-HRMS platform, the characterization of ADCs with different DARs can be realized
2.2 How to determine ADC DAR?
DAR analysis can be divided into two aspects. First is DAR distribution, which characterizes the proportion of ADC molecules with different DARs in the total ADC molecules. Second is the average DAR, which is the ratio of the molar concentration of the total ADC molecules to that of the antibody molecule in the system. WuXi AppTec DMPK has developed an LC-HRMS intact protein analysis to characterize the ADC DAR. Compared to HIC-UV and traditional DAR characterization methods, the LC-MS method can greatly reduce sample consumption and provide more accurate DAR results7.
For ADCs in a biomatrix, the general approach is to first use the anti-human IgG, idiotype antibody, or antigen that can specifically bind to the target ADC to conduct immuno-affinity capture for the target ADC. Next, the LC-HRMS platform is used to conduct direct analysis on the purified ADC, and finally, deconvolution software is utilized for data processing (Figure 3). The specific peaks of ADC molecules with different absolute molecular weights and different DARs are obtained to complete the DAR analysis of ADCs.
In addition, purified ADC can be broken down to separate the light and heavy chains and then undergo an LC-HRMS assay. Once the deconvolution of the spectrogram is acquired, DARs of light and heavy chains can be obtained. The DAR analysis of ADCs can then be performed indirectly.

Figure 3. ADC DAR characterization process
This platform is used to verify the in vitro/in vivo stability of the T-DM1 of ADCs. It provides a DAR analysis method with ultra-high sensitivity (LLOQ: 0.5-1 μg/mL), specificity, and accuracy. The in vitro stability study results are shown in Figure 4. As seen below, the intact protein analysis results revealed the variation of DARs of T-DM1.

Figure 4. In vitro plasma stability of T-DM1. With the increase in incubation time, the content of high-DAR ADCs (DAR6 in the figure disappeared after 24 h) gradually decreased, and the average DAR of ADC saw a corresponding decrease from 3.34 at T0 to 2.21 after 48 hours
3. Quantitative Bioanalysis of ADC Drug Components in Serum/plasma or Other Biological Matrices
ELISA (enzyme-linked immunosorbent assay), which is based on the LBA platform, is typically regarded as the “gold standard” in macromolecular quantitative research. However, ELISA usually needs to be optimized for different base species and matrix types. The universal reagents and methods are not ideal and the method development cycle is generally long. These aspects are particularly prominent for the early development of ADCs3. In recent years, with the continuous development of mass spectrometry, LC-MS/MS and LC-HRMS are increasingly used for macromolecular characterization and quantification4–6. Compared with the traditional ELISA, LC-MS offers a shorter development cycle and better standardization. In addition, LC-HRMS based on high-resolution mass spectrometry not only can quantify macromolecules but also determine the DARs of ADCs, thus facilitating the improvement of ADCs’ biological characterization.
ADCs are often present in vivo as complex and dynamically changing mixtures caused by biotransformation, DAR changes, or a combination of these processes. In this case, the existing PK is no longer clear with regards to the expression of “therapeutic concentration” and time, which presents a unique challenge to quantitatively analyzing ADCs. As shown in Table 1, the components for analysis currently available to characterize ADC PK include total antibody (an antibody that is conjugated or unconjugated to the payload), conjugated antibody (ADC, antibody that is conjugated to at least one payload and is a prototype drug of ADC), unconjugated payload, conjugated payload and payload related species (effector formed after ADC cleavage or decomposition)3.
The PKs of different analytes reflect different content and significance, presenting the complete picture of ADC metabolism in vivo. Therefore, a comprehensive LC-MS platform is needed for the PK analysis of total antibodies, intact ADCs, unconjugated payload, and conjugated payload.
3.1 Quantitative analysis of total antibody
For total antibody analysis of ADCs, sample preparation, and analysis remain basically consistent with other antibody drugs. Usually, the process can be divided into three steps (Figure 5):
Immuno-affinity capture, which involves antibodies or antigens that are specifically bound to the ADC antibody to purify the target total antibody.
Proteolysis, which generates the signature peptides of the antibody.
LC-MS /MS, which is used for quantitative determination.
The determination of total antibody is quite consistent with the first step of DAR analysis. The only difference is that when the LC-MS/MS platform is used to determine the total antibody, the requirements for the specificity of the capture reagents are reduced, meaning that a wide range of options are available to reduce the cost. Beyond anti-human IgG, idiotype antibody, and antigen, we can also use Protein A/G which has better universality, poorer specificity, and lower price. WuXi AppTec DMPK has carried out substantial verification and optimization for the above types of capture reagents and various types of antibody structures through numerous projects, which successfully run your projects and reduce turnaround time.

Figure 5. LC-MS/MS quantification process of total antibody
3.2 Quantitative ADC bioanalysis
It is important to begin this section by defining the target analyte of ADC quantitative analysis. There are generally two ways to define the PK target analyte of ADCs:
From the perspective of antibodies, it can be defined as antibody molecules bound to at least one drug.
From the perspective of drug load, it can be defined as the total concentration of the drug bound to the antibody.
From the two definitions above, we have developed two different bioanalysis strategies.
If an antibody molecule bound to more than one drug is used as the analyte, we can use a method similar
to total antibody analysis. The difference is that the capture reagent used in the first step must be an anti-drug antibody. Therefore, the antibody molecules conjugated to drugs can be extracted and quantified using LC-MS/MS4. If the drug conjugated to the antibody is used as the analyte, and the linker can be cleaved through sample processing to release the drug, then the conjugated drug can be released after the purified total antibody is captured (Figure 6). Furthermore, the concentration of the conjugated drug can be determined to achieve the quantitative ADC bioanalysis.
In addition to the two situations noted above, through the steady development of site-specific conjugation8,the “payload-linker-amino acid” generated by proteolysis has also been used as the analyte for ADC quantitative analysis in recent years. This makes it possible to achieve the quantitative analysis of conjugated payload. Theoretically, the average DAR of ADCs can be indirectly detected through the analysis of the quantitative results of conjugated payload and total antibody.

Figure 6. Quantitative strategy for ADC: 1) For non-cleavable linkers, the ADC can be captured by using an anti-payload antibody and the antibody molecules conjugated to the drug can be quantified; 2) For cleavable linkers, the total concentration of the drug bound to the antibody can be quantified by releasing conjugated payload.
3.3 LC-MS/MS quantitative analysis of free payload
In the production process of ADCs, it is often necessary to eliminate excess payload to the greatest extent possible. Due to its ultra-high toxicity, even a very small quantity of residues will also bring great potential dangers to ADC safety.
For in vivo studies, the unconjugated payload may come from the release of ADC in plasma and target tissues. The unconjugated payload in plasma corresponds to the off-target ADC toxicity and serves as an important part of the performance evaluation of ADCs.
The unconjugated payload of ADCs has significant potential toxicity. WuXi AppTec DMPK has been equipped with a pre-treatment room of OEB4 level, which can maximize the protection of the safety of laboratory personnel. For conventional payloads such as MMAE, MMAF, DM1, and SN-38, the quantitative linear range of 1-3000 ng/ mL can be achieved only by using a simple protein precipitation method. For special payloads, if the conventional protein precipitation method cannot meet the quantitative requirements, our team has experienced experts in small molecular analysis to optimize LLE, SPE, and other small molecule extraction methods.
Conclusion
The ADC analysis team of WuXi AppTec DMPK has established a qualitative/semi-quantitative analysis method for intact ADCs from plasma/serum or other matrices, which can detect the change in in vivo and in vitro ADC DAR and possible biotransformation. In addition, there is a mature and complete quantitative analysis protocol for various ADC components that can partially represent the PK characteristics, including total antibody, ADC, unconjugated payload, and conjugated payload (Figure 7). The intact ADC analysis method based on high-resolution mass spectrometry can even provide extremely extensive PK characteristic parameters of ADCs in just a single detection, including concentration and DAR. All these methods collectively form a complete set of ADC bioanalysis strategies. Combined with DMPK's professional team of large and small animals, we provide customers with a one-stop ADC PK and bioanalysis strategy, making the metabolism and pharmacokinetics analysis of ADCs significantly easier.

Figure 7. Summary diagram of LC-MS assay strategy for ADC related components
Click here to learn more about the strategies for ADC, or talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Author: Jianhui Cheng, Zhiyu Li
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,500+ global clients, and have successfully supported 1,200+ IND applications.
Reference
[1] Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Linkers Having a Crucial Role in Antibody-Drug Conjugates. Int. J. Mol. Sci. 2016, 17 (4), 561. https: //doi.org/10.3390/ijms17040561.
[2] Full article: Targeting cancer with antibody-drug conjugates: Promises and challenges https: //www.tandfonline.com/doi/full/10.1080/19420862.2021.1951427 (accessed 2021 -10 -23).
[3] Zhu, X.; Huo, S.; Xue, C.; An, B.; Qu, J. Current LC-MS-Based Strategies for Characterization and Quantification of Antibody-Drug Conjugates. J. Pharm. Anal. 2020, 10 (3), 209–220. https: //doi.org/10.1016/j.jpha.2020.05.008.
[4] Huang, Y.; Mou, S.; Wang, Y.; Mu, R.; Liang, M.; Rosenbaum, A. I. Characterization of Antibody–Drug Conjugate Pharmacokinetics and in Vivo Biotransformation Using Quantitative Intact LC-HRMS and Surrogate Analyte LC-MRM. Anal. Chem. 2021, 93 (15), 6135–6144. https: //doi.org/10.1021/acs.analchem.0c05376.
[5] Jin, W.; Burton, L.; Moore, I. LC-HRMS Quantitation of Intact Antibody Drug Conjugate Trastuzumab Emtansine from Rat Plasma. Bioanalysis 2018, 10 (11), 851–862. https: //doi.org/10.4155/bio-2018-0003.
[6] Kellie, J. F.; Pannullo, K. E.; Li, Y.; Fraley, K.; Mayer, A.; Sychterz, C. J.; Szapacs, M. E.; Karlinsey, M. Z. Antibody Subunit LC-MS Analysis for Pharmacokinetic and Biotransformation Determination from In-Life Studies for Complex Biotherapeutics. Anal. Chem. 2020, 92 (12), 8268–8277. https: //doi.org/10.1021/acs.analchem.0c00520.
[7] Källsten, M.; Hartmann, R.; Artemenko, K.; Lind, S. B.; Lehmann, F.; Bergquist, J. Qualitative Analysis of Antibody–Drug Conjugates (ADCs): An Experimental Comparison of Analytical Techniques of Cysteine-Linked ADCs. Analyst 2018, 143 (22), 5487–5496. https: //doi.org/10.1039/C8AN01178H.
[8] Lee, B. ill; Park, M.-H.; Byeon, J.-J.; Shin, S.-H.; Choi, J.; Park, Y.; Park, Y.-H.; Chae, J.; Shin, Y. G. Quantification of an Antibody-Conjugated Drug in Fat Plasma by an Affinity Capture LC-MS/MS Method for a Novel Prenyl Transferase-Mediated Site-Specific Antibody–Drug Conjugate. Molecules 2020, 25 (7), 1515. https: //doi.org/10.3390/molecules25071515.
Related Services and Platforms




-

 DMPK BioanalysisLearn More
DMPK BioanalysisLearn More -

 Novel Drug Modalities DMPK Enabling PlatformsLearn More
Novel Drug Modalities DMPK Enabling PlatformsLearn More -

 Novel Drug Modalities BioanalysisLearn More
Novel Drug Modalities BioanalysisLearn More -

 Small Molecules BioanalysisLearn More
Small Molecules BioanalysisLearn More -

 Bioanalytical Instrument PlatformLearn More
Bioanalytical Instrument PlatformLearn More -

 PROTAC DMPK ServicesLearn More
PROTAC DMPK ServicesLearn More -

 ADC DMPK ServicesLearn More
ADC DMPK ServicesLearn More -

 Oligo DMPK ServicesLearn More
Oligo DMPK ServicesLearn More -

 PDC DMPK ServicesLearn More
PDC DMPK ServicesLearn More -

 Peptide DMPK ServicesLearn More
Peptide DMPK ServicesLearn More -

 mRNA DMPK ServicesLearn More
mRNA DMPK ServicesLearn More -

 Covalent Drugs DMPK ServicesLearn More
Covalent Drugs DMPK ServicesLearn More
Stay Connected
Keep up with the latest news and insights.