Since 2018, the FDA has approved over ten new oligonucleotide drugs, highlighting the rapid emergence of oligonucleotide therapeutics as a promising drug modality. A critical aspect of developing these therapies is their metabolic stability, which necessitates the selection of appropriate in vitro metabolism models for thorough investigations. However, many existing in vitro models often fail to predict in vivo metabolism, leading to potential discrepancies in therapeutic efficacy and safety.
To address this challenge, there is a growing need for in vitro models that can effectively simulate the complex metabolic environments found in vivo. Such models are essential for conducting comprehensive metabolic stability assessments and identifying metabolites that may influence the pharmacokinetics and pharmacodynamics of oligonucleotide therapies.
In our systematic study, we focus on developing suitable in vitro metabolic systems and metabolite identification strategies specifically tailored for oligonucleotides. Through this talk, we aim to share our insights and methodologies designed to facilitate the optimization and development of oligonucleotide therapeutics, ultimately enhancing their therapeutic potential and accelerating their translation into clinical practice.
Learning Objectives:
The current trend in the development of oligonucleotide drugs.
The Importance of in vitro metabolic systems for oligonucleotides.
How to choose suitable in vitro models to perform metabolic stability assessment and metabolite profiling, and identification of oligonucleotides.
Strategies for oligonucleotide metabolite identification.
Speaker
Hong Zhang, Ph.D.
Dr. Hong Zhang, Ph.D. of Pharmacy, is mainly responsible for the metabolite profiling and identification of oligonucleotides in the DMPK Service Department of WuXi AppTec. He has over 10 years of working experience in the metabolite identification by LC-HRMS and extensive experience in the preclinical development of drugs. Dr. Zhang worked as Associate Professor in School of Pharmacy, Shanghai University of Traditional Chinese Medicine prior to joining WuXi AppTec. He has authored over 40 peer-reviewed papers in internationally renowned journals, including Pharmacological Research, Analytica Chimica Acta, Journal of Chromatography A, and Journal of Natural Products, among others.
Related Resources
-


Deciphering Nucleoside and Analogs: Mechanisms, Landscape, and Their Phosphates Analysis based on LC-MS/MS
ArticlesFeb 27,2026 -


What Are Antibody–Oligonucleotide Conjugates (AOCs) and Their Structural Characteristics
ArticlesNov 28,2025 -


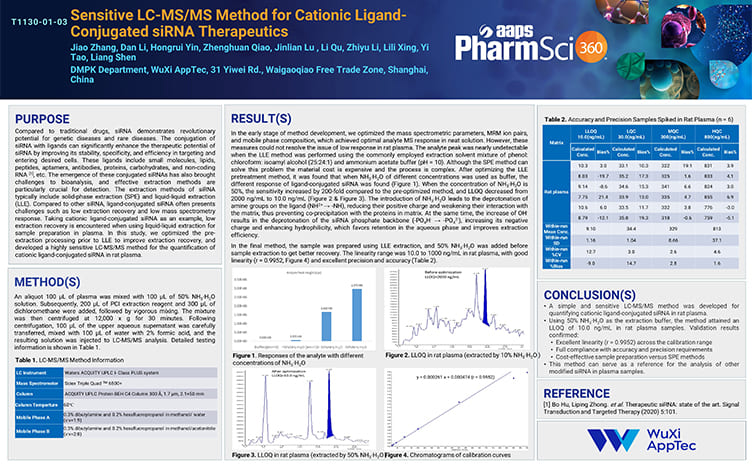
Sensitive LC-MS/MS Method for Cationic Ligand-Conjugated siRNA Therapeutics
PostersNov 08,2025
Related Services and Platforms




-

 In Vitro ADME ServicesLearn More
In Vitro ADME ServicesLearn More -

 Novel Drug Modalities DMPK Enabling PlatformsLearn More
Novel Drug Modalities DMPK Enabling PlatformsLearn More -

 Physicochemical Property StudyLearn More
Physicochemical Property StudyLearn More -

 Permeability and Transporter StudyLearn More
Permeability and Transporter StudyLearn More -

 Drug Distribution and Protein Binding StudiesLearn More
Drug Distribution and Protein Binding StudiesLearn More -

 Metabolic Stability StudyLearn More
Metabolic Stability StudyLearn More -

 Drug Interactions StudyLearn More
Drug Interactions StudyLearn More
Stay Connected
Keep up with the latest news and insights.