Peptide drugs are known for their high specificity, strong target affinity, and low risk of off-target effects. However, compared to small molecule drugs, they suffer from low oral bioavailability, poor plasma stability, and short circulation time. Optimizing these DMPK (Drug Metabolism and Pharmacokinetics) properties of peptides is crucial for their development. This webinar will explore essential strategies for optimizing the DMPK profiles of peptides, including the selection of appropriate in vitro matrices, tailored sample processing techniques, and leveraging WuXi AppTec’s extensive experience and study platforms. Attendees will gain insights into the key factors that influence peptide drug development, from early screening to IND-enabling studies.
Learning objectives:
Understand the specific DMPK properties and challenges associated with peptide drugs.
Explore strategies to improve the stability, permeability, and bioavailability of peptide drugs.
Understand the comprehensive DMPK approaches for successful peptide drug development, from screening to IND submission.
Related Resources
-


Decoding Peptide ADME Challenges: Integrated Radiolabeled Synthesis & ADME Platform - DMPK Frontiers
VideosOct 24,2025 -


In Vitro Metabolic Stability of Peptide Drugs Across Different Tissues
ArticlesSep 30,2025 -


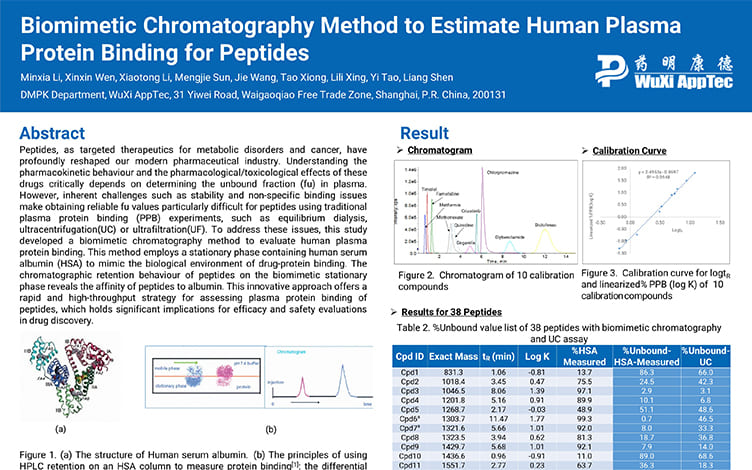
Biomimetic Chromatography Method to Estimate Human Plasma Protein Binding for Peptides
PostersSep 19,2025
Related Services and Platforms




Stay Connected
Keep up with the latest news and insights.