On May 15, 2023, WuXi AppTec DMPK Nantong R&D center officially opened. The Nantong R&D center will focus on conducting Non-rodent (Large Animal) PK Studies of European standards and Non-GLP bioanalytical research services. The animal species include dogs, monkeys, mini-pigs, rabbits, and ferrets and studies cover stages from early development to IND (Investigational New Drug) filing.

Figure 1. WuXi AppTec DMPK Nantong R&D center
The Nantong R&D center holds an R&D laboratory with an area of over 18,000 square meters, Non-rodent facilities of international standards & European standards, and a high-precision and high-throughput Non-GLP bioanalysis laboratory. The R&D center has implemented comprehensive digital operations to ensure every customer project is delivered efficiently and safely. In addition, the center is equipped with pleasant office and rest environments to create a comfortable working atmosphere for employees.

Figure 2. Laboratory and office environments of Nantong R&D center
International Standard Non-rodent PK Research Services
The Nantong R&D center can accommodate thousands of large animals including dogs, monkeys, pigs, rabbits, and ferrets, which meet different standards of China, the United States, and Europe. Different types of projects, including normal screening PK, Dose Range Finding (DRF), and Investigational New Drug (IND) applications, etc. can be conducted in the center.
Species | Strains | Types of projects |
Dog | Beagle | Screening PK, IND application |
Monkey | Cynomolgus, Rhesus | Screening PK, IND application |
Pig | Bama Mini-pig | Transdermal administration |
Rabbit | New Zealand White, Dutch | Ocular PK |
Ferret | Ferret | Influenza virus-related studies |
Table 1. Animal species and the corresponding project types commonly conducted.
(1) Environment
The animal facility is equipped with a HEPA-filtered and HVAC system. WebCTRL environment control system is applied to monitor the temperature, relative humidity, and pressure difference within the animal rooms. 12-hour light/dark cycles are automatically controlled for animal holding rooms. These environmental parameters meet international standards, including Chinese, American, and European standards. Moreover, animal environment enrichments, including toys, fruits, music, and TV are also provided for different species to maintain their physiological and psychological health.

Figure 3. Animal welfare support facilities
(2) Non-rodent surgery and special capabilities
The non-rodent PK team has abundant surgical, specialized operations, and veterinary clinical experience, and can provide the capabilities of surgical cannulation models, administration routes, and special sample collection services. It refers to the system of central nervous, respiratory, immune, alimentary, metabolic, motor nervous, urogenital, etc.
Surgical models include cannulation in cisterna magna, hepatic portal vein, bile duct, intestine, thoracic duct (or lymphatic duct), lateral ventricle, femoral artery, and femoral vein. These models allow continuous and multiple sample collection or long-term drug administration in conscious animals. The team is also capable of intrathecal administration, B-ultrasound-guided livers, and kidney biopsies (Figure 4) to assist specific targeted purposes in drug development.
Other special operations include sublingual administration, over 24-hour IV infusion, intraocular administration followed by collection and isolation of various ocular tissues, transdermal administration followed by skin collection, and sampling of various tissues.

Figure 4. Large animal surgical models and specialized operating techniques
(3) Clinical pathology laboratory
The DMPK Services Department’s clinical pathology laboratory can provide capabilities including clinical chemistry, CBC (complete blood count), coagulation, and urinalysis. The laboratory instruments and equipment (Figure 5) offer stable performance and high throughput screening with fast speed. The laboratory also offers morphological examination capabilities including urine sediment and blood smear testing.
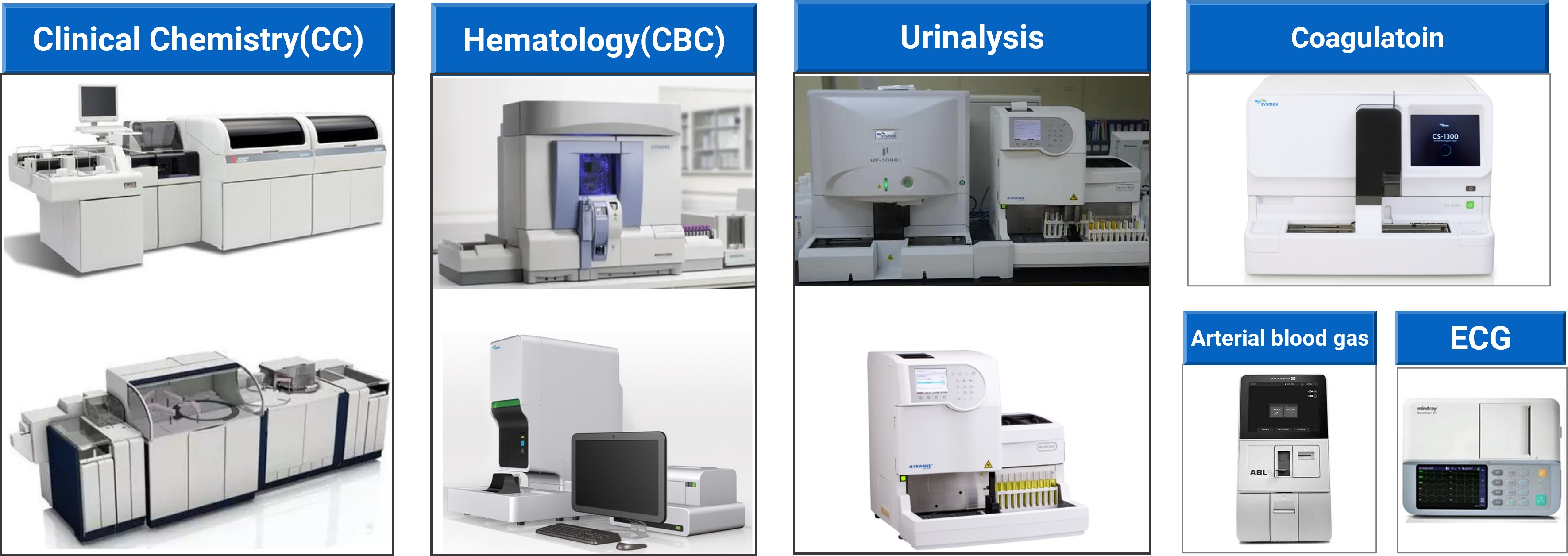
Figure 5. Clinical pathology laboratory equipment
Non-GLP Bioanalytical Platform
Based on professional analytical talents, extensive analytical experience, high-precision, and high-throughput analytical equipment, comprehensive digital project management, and reliable quality control systems, DMPK Nantong R&D Center is capable of providing efficient and high-quality Non-GLP bioanalytical services throughout all pre-clinical stages of drug development, helping customers to solve the increasingly complex bioanalysis challenges of various molecular entities.
The R&D center of the Nantong site has the analytical capability for over a hundred special matrices, including cerebrospinal fluid, microdialysate, PBMC, and ocular tissue. Additionally, they have established standardized experimental procedures for microvolume samples, such as dried blood spot, dried plasma spot, and volumetric absorptive microsampling (VAMS), covering the entire process from sample collection to bioanalysis. The platform is capable of analyzing a wide type of test articles, including conventional small molecules, biomarkers, Proteolysis-Targeting Chimeras (PROTACs), peptides, liposomes, ADCs, PDCs, proteins, and nucleic acids.

Figure 6. Integrated Non-GLP bioanalytical services
A Final Word
The launch of the WuXi AppTec DMPK Nantong R&D center has expanded the capabilities and capacities of the DMPK Services Department, further ensuring the sustainability of the business. At this point, DMPK Department has established 5 R&D centers (Shanghai, Suzhou, Nanjing, Nantong, and New Jersey) worldwide, providing comprehensive and high-standard PK study services. In the future, we will continue to expand the department's capability and capacity to accelerate clients’ new drug development processes.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,500+ global clients, and have successfully supported 1,200+ IND applications.
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Stay Connected
Keep up with the latest news and insights.



