Join us October 22-25 at the Orange County Convention Center, Orlando FL for the world's premier gathering of pharmaceutical scientists.
Featured Presentation
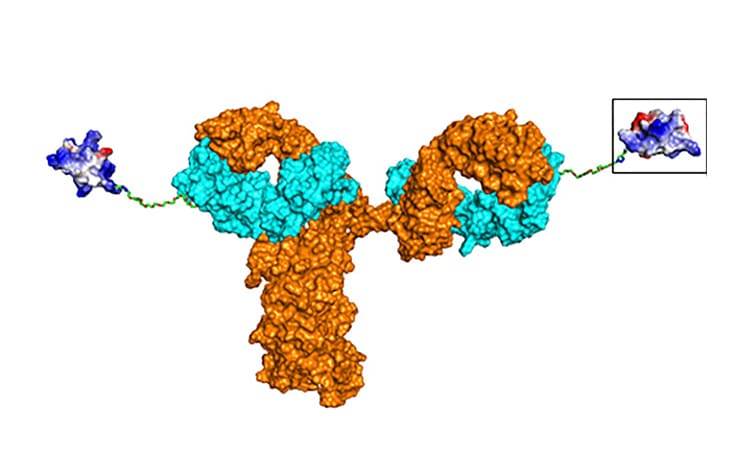
Integrated Bioanalysis and Biotransformation Strategies for Antibody-Drug Conjugates
Li Qu, Ph.D. | Deputy Director, Non-GLP Bioanalysis, WuXi AppTec
Peng Li, Ph.D. | Senior Scientist, MetID, WuXi AppTec
This presentation will explore the comprehensive evaluation of Antibody-Drug Conjugates (ADCs) through integrated bioanalysis and advanced biotransformation studies. Key topics include nonclinical pharmacokinetic assessment, metabolite profiling, Drug-to-Antibody Ratio (DAR) analysis, and strategies to address ADC complexities throughout development. Attendees will gain insights into leveraging multiple analytical platforms and hybrid methods to overcome challenges in ADC drug development, facilitating the ADC drug R&D process.
Learning Objectives:
Understand the role of integrated bioanalysis in nonclinical pharmacokinetic evaluation of ADCs.
Learn advanced methodologies for ADC biotransformation, including metabolite profiling and DAR analysis.
Explore strategies to overcome challenges in ADC development using comprehensive analytical approaches.
Posters
Other Resources
-
Posters
-
Other Resources
-
Simultaneous Determination of Metabolites from Seven Key Probe Substrates in CYP Inhibition Assay Using Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)Development of a Rapid, High Throughput Quantitative Bioanalysis Method for Conjugated Payloads of Protease-Cleavable Antibody-Drug Conjugate (ADC) in Rat Serum Using Automated Immuno-Capture Based UPLC-MS/MSView More
-
-


A Review on ADC Linkers: Cleavable and Non-Cleavable Linker Design and Stability Evaluation
ArticlesJan 30,2026 -


What Are Antibody–Peptide Conjugates (APCs) Drugs and Their Pharmacokinetic Properties
ArticlesNov 21,2025 -


Integrated Bioanalytical Platform and DMPK Strategies for Antibody-Drug Conjugates
WebinarsNov 13,2025
-
Related Services and Platforms




-

 MetID (Metabolite Profiling and Identification)Learn More
MetID (Metabolite Profiling and Identification)Learn More -

 Novel Drug Modalities DMPK Enabling PlatformsLearn More
Novel Drug Modalities DMPK Enabling PlatformsLearn More -

 In Vitro MetID (Metabolite Profiling and Identification)Learn More
In Vitro MetID (Metabolite Profiling and Identification)Learn More -

 In Vivo MetID (Metabolite Profiling and Identification)Learn More
In Vivo MetID (Metabolite Profiling and Identification)Learn More -

 Metabolite Biosynthesis and Structural CharacterizationLearn More
Metabolite Biosynthesis and Structural CharacterizationLearn More -

 Metabolites in Safety Testing (MIST)Learn More
Metabolites in Safety Testing (MIST)Learn More -

 PROTAC DMPK ServicesLearn More
PROTAC DMPK ServicesLearn More -

 ADC DMPK ServicesLearn More
ADC DMPK ServicesLearn More -

 Oligo DMPK ServicesLearn More
Oligo DMPK ServicesLearn More -

 PDC DMPK ServicesLearn More
PDC DMPK ServicesLearn More -

 Peptide DMPK ServicesLearn More
Peptide DMPK ServicesLearn More -

 mRNA DMPK ServicesLearn More
mRNA DMPK ServicesLearn More -

 Covalent Drugs DMPK ServicesLearn More
Covalent Drugs DMPK ServicesLearn More
Stay Connected
Keep up with the latest news and insights.
Submission successful!
We will send you an email with all the posters from this conference once they are ready. Thank you!
-
ok