Central nervous system (CNS) disorders—such as Alzheimer’s disease (AD), Parkinson’s disease (PD), epilepsy, and stroke, are among the most debilitating neurological disorders worldwide. Affecting 1 in 8 people globally, these disorders impair cognitive function, motor skills, and overall quality of life. The World Health Organization (WHO) highlights dementia as the fifth leading cause of death, with AD alone accounting for 60-70% of cases, underscoring the urgent need for improved therapeutic strategies. Yet CNS drug development faces a daunting success rate of less than 10%, predominantly due to the brain’s formidable defense system: the blood-brain barrier (BBB). This selective barrier blocks over 98% of small-molecule drugs and nearly all biologics, creating a bottleneck for effective treatments. However, advancements in pharmacokinetic research are shedding light on innovative strategies to bypass these barriers, offering hope for breakthroughs in central nervous system drugs.
What Is the Blood-Brain Barrier and How Does It Work?
In addition to the physical protection provided by the skull, the brain is protected by a special biological barrier, including the BBB, blood-cerebrospinal fluid barrier (BCSFB), and cerebrospinal fluid-brain barrier (CSF-brain barrier) (Figure 1), among which the BBB has the lowest permeability. The poor permeability and the function of the BBB arise from its structure: tightly packed endothelial cells, supported by astrocytes and pericytes, shield the brain from toxins and pathogens. Unlike the peripheral nervous system, which transmits signals throughout the body, the CNS relies on the BBB to maintain a stable environment for neural function.

Figure 1. A Three-Compartment Model Illustrates Unbound Drug Distribution Equilibrium Between Plasma, Brain (ISF and ICF), and CSF[1]
How Do Substances Cross the Blood-Brain Barrier?
Substances cross the blood-brain barrier through six primary pathways: passive diffusion, transporter-mediated uptake, receptor-mediated transcytosis, paracellular transport, efflux transporters, and temporary disruption techniques (Figure 2). Passive diffusion and paracellular transport pathways are limited to small hydrophobic and hydrophilic molecules, respectively. Transporter-mediated uptake enables nutrients like glucose to enter the brain via specific carriers. Biologic drugs often exploit receptor-mediated transcytosis, “hijacking” receptors like transferrin to hitchhike into the brain. However, Substances that enter the brain via various ways may be excreted by efflux transporters.

Figure 2. The Way Substances Enter and Leave the Blood-Brain Barrier[2]
BBB Permeability Evaluation: In Vivo and In Vitro Assays
#1 In Vitro Permeability and Transporter Evaluation Assays
Validating a drug’s ability to cross the blood-brain barrier requires a combination of in vitro and in vivo models. High-throughput assays like BBB-PAMPA simulate passive diffusion using artificial membranes coated with brain lipids, while MDR1-MDCK cell models identify compounds susceptible to P-gp efflux.
To verify the accuracy of BBB-PAMPA and MDR1-MDCK I models in predicting brain penetration, 24 commercial compounds, including 10 BBB permeable (CNS+) compounds and 14 BBB impermeable compounds (CNS-) [3-7], were evaluated. In this two-tier screening approach, BBB-PAMPA was first employed to eliminate compounds with poor passive permeability, followed by MDR1-MDCK I to screen out P-gp efflux transporters' substrates. Notably, this sequential screening accurately identified all 10 CNS+ compounds (Figure 3). Based on these results, we proposed a “Funnel” model incorporating both assays for efficient early-stage screening of BBB-permeable compounds (Figure 4).

Figure 3. 10 CNS+ Compounds Were Selected Accurately (Green Square), Combining BBB-PAMPA and MDR1-MDCK I Assays

Figure 4. “Funnel” Model to Evaluate and Select BBB Permeable Compounds
#2 In Vivo Brain Penetration Evaluation Models
The in situ brain perfusion model is a sensitive method to evaluate BBB permeability. The perfusate containing the testing compound is perfused into the brain via the carotid artery. At the end of perfusion, the cerebral vessels are flushed with blank perfusate, and the brain is excised, homogenized, and analyzed to determine the compound concentration.
Brain microdialysis is another pivotal in vivo technique that enables real-time monitoring of drug pharmacokinetics. The method involves implanting a probe into the brain to measure free drug concentrations.
This approach allows researchers to monitor drug distribution between plasma, brain tissue, and cerebrospinal fluid (CSF). For example, the simultaneous collection of blood and microdialysate samples on the same animal enables direct calculation of Kp, uu, a critical parameter for evaluating BBB penetration. However, the technique has limitations: compounds with high non-specific binding may lead to unreliable results. Therefore, preliminary in vitro validation is required to confirm feasibility.

Figure 5. Brain Microdialysis Device and Probes
# 3 A Stepwise Screening Strategy for CNS Drugs
Reliable in vitro assessment models, physiologically relevant parameter thresholds, and robust screening methodologies are essential for selecting promising candidates. We have developed an integrated platform for the systematic evaluation of CNS drug properties. This stepwise screening strategy allows for rapid, accurate, and efficient assessments of CNS drugs.
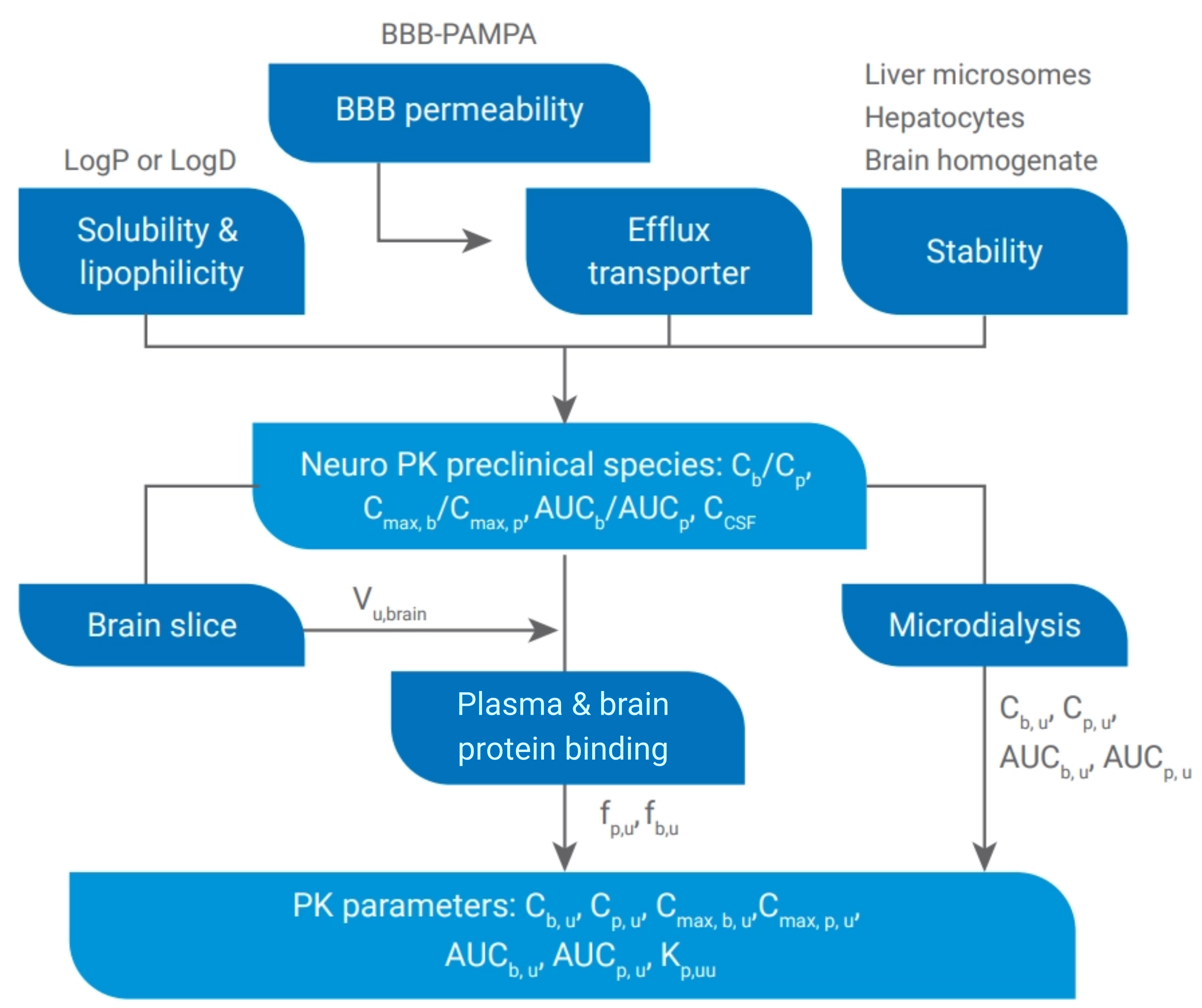
Figure 6. Screening Paradigm for CNS Drugs Selection and Optimization
Concluding Remarks
Developing effective CNS drugs requires a deep understanding of the BBB’s biology and the courage to challenge conventional paradigms. By integrating molecular structural optimization, advanced permeability assays like BBB-PAMPA, and real-time neuropharmacokinetic tools such as brain microdialysis, the pharmaceutical industry is making incremental progress towards transformative therapies for AD, PD, and other CNS disorders. For biologic drugs, the development of cutting-edge therapies is also progressing at an accelerated pace. Taking oligonucleotide (oligo) as an example, the transport vehicle (TV) facilitates receptor-mediated transcytosis across the blood-brain barrier, while intrathecal (IT) administration enables drugs to bypass the BBB and directly reach the CNS. As science continues to unravel the mysteries of the blood-brain barrier and CSF dynamics, the promise of healing the brain’s most complex ailments grows ever brighter.
Authors: Fengyun Shen, Tingting Ruan, Qing Liu, Tao Xiong, Xuan Dong, Cheng Tang, Genfu Chen, Jing Jin
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Di L, Rong H, Feng B. Demystifying brain penetration in central nervous system drug discovery. Miniperspective. J Med Chem. 2013 Jan 10; 56 (1): 2-12. doi: 10.1021/jm301297f. Epub 2012 Nov 6. PMID: 23075026.
[2] Georgieva, J.V.; Hoekstra, D.; Zuhorn, I.S. Smuggling Drugs into the Brain: An Overview of Ligands Targeting Transcytosis for Drug Delivery across the Blood – Brain Barrier. Pharmaceutics 2014, 6, 557-583.   http://doi.org/10.3390/pharmaceutics6040557
[3] Tsinman O, Tsinman K, Sun N, Avdeef A. Physicochemical selectivity of the BBB microenvironment governing passive diffusion-matching with a porcine brain lipid extract artificial membrane permeability model. Pharm Res. 2011 Feb; 28 (2): 337-63.  
[4] Drion, N et al. "Role of P ‐ Glycoprotein in the Blood ‐ Brain Transport of Colchicine and Vinblastine." Journal of Neurochemistry 67 (1996): n. pag.
[5] A.M. Karssen, O.C. Meijer, E.R. de Kloet, Chapter 3.4 - Corticosteroids and the blood-brain barrier, Editor (s): T. Steckler, N.H. Kalin, J.M.H.M. Reul, Techniques in the Behavioral and Neural Sciences, Elsevier, Volume 15, Part 1, 2005, Pages 329-340,
[6] Taskar KS, Mariappan TT, Kurawattimath V, Singh Gautam S, Radhakrishna Mullapudi TV, Sridhar SK, Kallem RR, Marathe P, Mandlekar S. Unmasking the Role of Uptake Transporters for Digoxin Uptake Across the Barriers of the Central Nervous System in Rat. J Cent Nerv Syst Dis. 2017 Mar 15; 9:1179573517693596. doi: 10.1177/1179573517693596. PMID: 28469522; PMCID: PMC5392048.
[7] Parkinson FE, Friesen J, Krizanac-Bengez L, Janigro D. Use of a three-dimensional in vitro model of the rat blood-brain barrier to assay nucleoside efflux from the brain. Brain Res. 2003 Aug 8; 980 (2): 233-41.
Stay Connected
Keep up with the latest news and insights.