Transporters are a class of membrane proteins, which play an essential role in drug absorption, distribution, and excretion. Reduced transporter expression or its deficiency can affect the drug disposition in the human body. In addition, the transporter may act as a potential target to induce drug-drug interactions (DDIs), ensuing altered pharmacokinetics and pharmacodynamics, and even cause drug toxicity[1]. Regulatory agencies, such as the FDA, EMA, and NMPA, recommended in vitro evaluation of potential interactions involving the primary transporters with the investigational new drugs. The purpose of this review is to systematically introduce the in vitro ABC (ATP-binding cassette) transporter research models, to facilitate the research and development (R&D) of new drugs.
What are ABC transporters
Transporters can be primarily divided into ABC transporters and solute carrier (SLC) transporters according to the different transport mechanisms[2]. ABC transporters, renowned for their dependence on the energy directly produced by adenosine triphosphatase (ATP) hydrolysis during substrate transport, have their ATP binding sites located intracellularly (Figure 1).

Figure 1. Schematic diagram of ABC transporter[3]
3 In vitro models of ABC transporters
The in vitro models currently used to evaluate DDI caused by ABC transporters primarily include the membrane vesicles, polarized cell monolayers, and the sandwich-cultured hepatocytes [4].
#1 Membrane vesicle model
Vesicles can be prepared from purified plasma membranes isolated from insect cells (e.g., Sf9 or Sf21) overexpressing ABC transporters, or by transfecting mammalian cells (e.g., HEK293, HeLa, V79 or MDCK (Madin-Darby canine kidney)). After everting the vesicles by a special process, the substrate binding site that is originally expressed inside of the vesicles will be everted to the outside, commonly known as " Inside-out " or " Inverted " membrane vesicles .
Since the substrate binding site is exposed outside from the inside-out vesicles, this configuration allows for direct interaction between the substrate and the ABC transporter, without the need for the drug to cross the membrane. In this case, ATP-dependent transport of the substrate could be measured when conducting DDI studies. Based on this characteristic, the membrane vesicle model could be used for the study of inhibition potential evaluation, substrate specificity, cofactor requirements, substrate affinity, etc. On the other hand, the effects of the compound on ABC transporter activity could be studied without the confounding effects of toxicity, since vesicles are not viable cells. However, it should be noted that false negative results might be obtained if the test compound has high permeability or strong non-specific binding , in this case, the compound may pass through the vesicle membrane or stick to it, leading to a high background that can mask the specific transport by the ABC transporter.

Figure 2. Schematic diagram of membrane vesicle model
#2 Polarized cell monolayer model
The polarized cell monolayer model refers to a model that employs a cell line capable of spontaneously polarizing to form an intact cell monolayer for TP-DDI (Transporter-related DDI) studies. Caco-2 cell line is the most commonly used cell model, and epithelial cell lines with overexpression of specific human transporters, such as Lewis lung carcinoma porcine kidney cell 1 (LLC-PK1) and MDCK, can also be used for related research.
A Transwell system is commonly used to assess the transport activity of chemicals. Cells are seeded onto a semi-permeable membrane of the transwell insert plates and cultured until a complete polarized cell monolayer is formed, by conducting bidirectional permeability (apical-basolateral (A‑B) and B-A directions) in the presence and absence of a specific ABC transporter inhibitor, the apparent permeability coefficient (Papp), as well as the efflux ratio (ER) and/or net flux ratio (NFR) could be calculated, thus evaluating whether the test compound is a substrate of the ABC transporter (Figure 3). In addition, an assessment of the inhibitory effect of a compound on a specific transporter could be performed by measuring the unidirectional flux of the probe substrate in the B-A direction or changes in ER values.
The bidirectional permeability assay of TP-DDI studies for ABC transporters using the transwell system requires attention to the following three points:
-
Recovery: If the test compound has strong non-specific binding or is easily retained in the cell, insufficient solution recovery may be observed (generally less than 70%). In addition, cellular metabolism should be noted since the incubation time of the polarized cell monolayers model is usually more than 60 minutes.
-
Transmembrane permeability: The substrate binding site of efflux transporter is located intracellularly in the polarized cell monolayers model, so the test compound needs to cross the membrane to interact with the substrate binding site. If the passive diffusion of the test compound is poor or it has limited ability to enter the cell through the mediation of the uptake transporter, the TP-DDI assessment would be complicated.
-
Test concentration: Bidirectional transport of the test compound in the transwell system is the superposition of the dual mechanisms of passive diffusion and active transport, where passive diffusion conforms to Fick's law of diffusion, and active transport complies with the Michaelis-Menten kinetics. If the concentration of the test compound is too high, the amount of compound transported passively can indeed overshadow those transported actively, which may mask the role of active transport. According to the Michaelis-Menten equation, the high concentration of the test compound would saturate the transporter binding site and potentially lead to a false negative result.
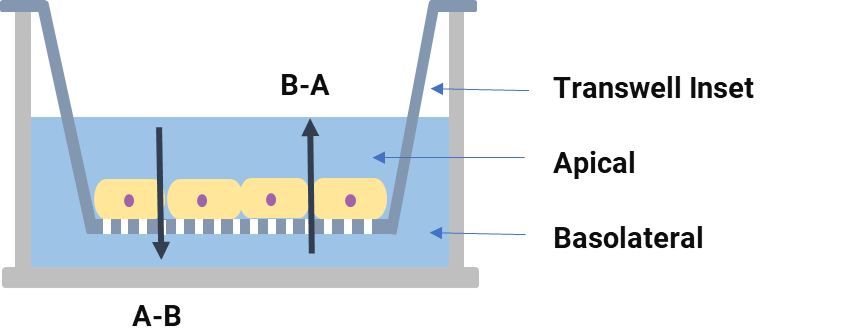
Figure 3. Schematic diagram of the Transwell system
It should be noted that when using Caco-2 cells to assess the substrate of ABC transporters, two or more specific inhibitors are recommended to be used to determine the specificity of the efflux since a variety of efflux transporters are expressed in the Caco-2 cells[5, 6]. In addition, when the test compound is a substrate for more than one efflux transporter, its efflux mediated by a specific transporter may be affected by others. The efflux may also compensatorily increase after the addition of a specific inhibitor of one efflux transporter, resulting in a false negative result.
#3 "Sandwich" cultured hepatocyte model
The method of culturing freshly isolated or cryopreserved hepatocytes on collagen-coated plates, then overlaying them with a secondary layer of collagen or matrigel, is referred to as the “sandwich” cultured hepatocyte model (Figure 4). After culturing in this system for a specific period (typically 4-6 days), hepatocytes undergo polarization and reconstruct the bile canalicular network. This network is an essential part of liver function as it helps in the secretion and transport of bile acids and other substances.
In “sandwich” cultured hepatocytes, the tight junctions of the bile canalicular network could be disrupted in a buffer containing ethylene glycol tetraacetic acid (EGTA). Therefore, the interaction between the compound and the bile canaliculi efflux transporter can be studied by comparing the accumulation of the compound in a normal buffer (representing cell + bile canalicular network content) and in a buffer containing EGTA (representing cellular accumulation) [7]. Under these conditions, whether the test compound is a substrate for a specific bile canaliculi efflux transporter could be assessed by adding the specific inhibitor.
However, only hepatocytes with high viability and high transporter expression could be used in the sandwich-cultured hepatocyte model, and the activities of intrahepatic metabolic enzymes and transporters are sensitive to the culture conditions. Meanwhile, while assessing the interaction of the test compound with bile canaliculi efflux transporters, hepatic metabolic enzymes also continue to function. Since the sandwich-cultured hepatocyte model requires a longer cultivation period, higher quality hepatocytes, and complex data interpretation, it is generally not used for early-stage high-throughput screening.
Each of the three in vitro models mentioned above has its advantages and limitations (Table 1). Researchers can select appropriate models for their studies based on the different application scenarios of each model.
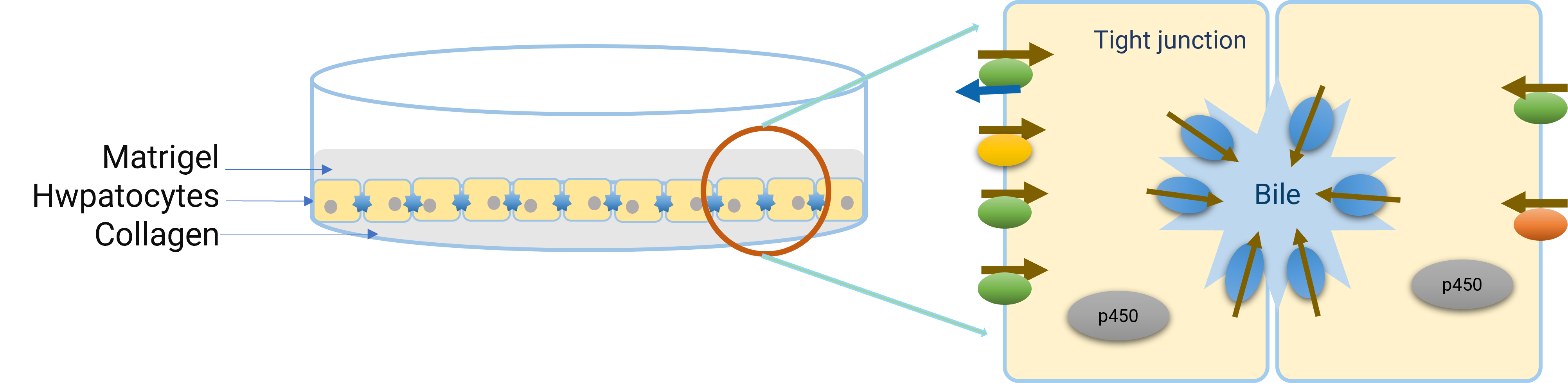
Figure 4. Diagram of a "Sandwich" cultured hepatocyte model
|
In Vitro Model |
Applications |
Advantages |
Limitations |
|
Membrane vesicles |
• Support substrate and inhibition assessment of ABC transporters |
• Easy to operate • Suitable for compounds with low permeability • Cytotoxic compounds do not impact the results • Accurate determination of kinetic parameters |
• Not suitable for high permeability or high non-specific binding compounds • Endogenous transport activity may complicate data interpretation • Transporter activity varies between batches |
|
Polarized cell monolayers |
• Available for substrate and inhibition assessment of ABC transporters |
• Low complexity • Evaluation of both active transport and passive diffusion • Multiple times sub-cultured or cryopreserved. • Simultaneously evaluate transporter substrate and predict human oral absorption (Caco-2 cells) |
• Long establishment period • Influence of endogenous transporters • Transporter expression levels vary between laboratories • Not suitable for compounds with poor transmembrane permeability. • Recovery needs to be assessed • Complicated kinetic studies |
|
"Sandwich" cultured hepatocytes |
• It can be used to study the efflux effect of bile canalicular efflux transporters as well as biliary excretion in vitro |
• Simulation of biliary excretion • Functionally intact with multiple factors • Availability for multiple species |
• Influence of culture conditions on transporter function • Complex data interpretation |
Table 1. Advantages and limitations of three in vitro ABC transporter models
Case study
The choice of research model should be based on the physicochemical properties of the drug itself and the purpose of the experiment. The following cases elaborate on the differences between using the vesicle model and the polarized cell monolayers model in studying TP-DDIs. In combination with the molecular types, a preliminary selection strategy of in vitro ABC transporter models is provided to assist drug R&D.
Evaluation of ABC transporter Inhibition and Substrate for Compounds with Poor Transmembrane Capabilities.
Evaluation of ABC transporter inhibition
The inhibitory effect of Compound A (poor permeability) on P‑gp was evaluated in the Caco-2 cell model and the MDR1 membrane vesicle model. The results are shown in Figure 5.

Figure 5. Assessment of P-gp inhibition by compound A in the Caco-2 cells model (a) and the MDR1 vesicle model (b)
Compound A may not be able to achieve a sufficient intracellular concentration in Caco-2 cells to fully inhibit P-glycoprotein (P-gp) due to its poor transmembrane permeability. In contrast, in the MDR1 membrane vesicle model, Compound A can directly interact with the P-gp binding site. This model bypasses the need for the compound to permeate the cell membrane. Therefore, despite its poor transmembrane permeability, Compound A can still exert its inhibitory effect on P-gp in the MDR1 membrane vesicle model.
For a drug to exert its inhibitory effect on ABC transporters in vivo, it needs to enter the cell and contact the transporter binding site. However, the extent to which a drug enters cells in vivo is influenced by multiple factors, such as the mediation of receptors or uptake transporters on the surface of intracellular tissues. It is difficult in the polarized cell monolayers model in vitro to evaluate the inhibitory effect of compounds with poor transmembrane permeability on ABC transporters. Therefore, the results may not be very informative.
Although using the membrane vesicles provides a realistic inhibition effect caused by free drug concentration near the substrate binding site, it might overestimate the drug's inhibition on ABC transporters. Given that we should aim to obtain the lowest possible half-maximal inhibitory concentration (IC50) in vitro to avoid potential clinical DDIs, the inhibitory results obtained in the vesicle model are more instructive in predicting whether a TP-DDIs could occur in vivo. Certainly, the data should be interpreted in the context of the model used. Therefore, for compounds with poor transmembrane permeability, it is recommended to use the membrane vesicle model to evaluate their inhibitory effect on ABC transporters.
Evaluation of ABC transporter substrate
Evaluations were conducted on whether Compound B is a BCRP substrate in both the Caco-2 cells model and the BCRP membrane vesicle model, with results as shown in Table 2. The two models yielded opposite conclusions.
|
Compound ID |
Compound B |
|||||
|
Model |
Caco-2 cells model |
BCRP membrane vesicle model |
||||
|
Parameter |
+/-BCRP inhibitor |
Papp (10-6 cm/s) |
Efflux ratio |
Uptake Fold |
Rs/Ri |
|
|
A-B |
B-A |
|||||
|
- |
0.0146 |
0.0221 |
1.51 |
30.3 |
53.6 |
|
|
+ |
0.0245 |
0.0213 |
1.15 |
|||
|
Conclusion |
Compound B was a poor or non-BCRP substrate |
Compound B was probably a BCRP substrate |
||||
Table 2. Evaluation of BCRP substrate for compound B in Caco-2 cells model and BCRP membrane
Note: Uptake Fold means the ratio of transport activity with ATP and AMP; Rs/Ri means the ratio of transport activity with ATP in the absence and presence of the inhibitor.
Due to the differences between in vivo and in vitro, compounds with poor transmembrane permeability struggle to cross the membrane and enter into cells in an in vitro polarized cell monolayer model. This does not necessarily reflect the real situation in vivo. Evaluating whether compounds with poor transmembrane permeability are substrates for ABC transporters using the membrane vesicle model can provide more instructive guidance on whether they could potentially cause clinical TP-DDIs. Therefore, the membrane vesicle model is more suitable for compounds with poor permeability when evaluating ABC transporter substrates.
Evaluation of ABC transporter substrate for highly permeable compounds
Evaluations were conducted on whether Compound C is a BCRP substrate in both the Caco-2 cells model and the BCRP membrane vesicle model. The results, as shown in Table 3, indicated that the two models yielded opposite conclusions.
|
Compound ID |
Compound C |
|||||
|
Model |
Caco-2 cell model |
BCRP membrane vesicle model |
||||
|
Parameter |
+/-BCRP inhibitor |
Papp (10-6 cm/s) |
Efflux ratio |
Uptake Fold |
Rs/Ri |
|
|
A-B |
B-A |
|||||
|
- |
7.14 |
31.3 |
4.38 |
1.52 |
1.53 |
|
|
+ |
15.6 |
17.9 |
1.15 |
|||
|
Conclusion |
Compound C was probably a BCRP substrate |
Compound C was a poor or non-BCRP substrate |
||||
Table 3. Evaluation of BCRP substrate for Compound C in Caco-2 cell model and BCRP membrane vesicle model
In the membrane vesicle model, the passive diffusion of Compound C, a highly permeable compound, into the vesicles creates a high background, which will mask the BCRP-mediated uptake, leading to a false-negative. Therefore, when evaluating whether high permeability compounds are substrates for ABC transporters using the vesicle model, false-negative results may be obtained. For such compounds, the polarized cell monolayers model is recommended.
Concluding remarks
By understanding the molecular characteristics and the differences in in vitro ABC transporter evaluation models, researchers can make more informed decisions about which model to use in their drug R&D. Choosing the appropriate model can yield more meaningful data, shorten the drug development cycle, and increase the success rate of drug development.
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Authors: Gongfang Shi, Qing Liu, Yajuan Bi, Tao Xiong, Genfu Chen
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, and clinical drug metabolism and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,500+ IND applications.
Reference
[1] Storelli, F., Yin, M., Kumar, A. R., et al. (2022). The next frontier in ADME science: Predicting transporter-based drug disposition, tissue concentrations and drug-drug interactions in humans. Pharmacology & Therapeutics, 238, 108271: 1-30.
[2] You G, Morris M E. (2006). Drug Transporters: Molecular Characterization and Role in Drug Disposition. New york: John Wiley & Sons, Inc.
[3] Reactome. “Disorders of transmembrane transporters.” Accessed Dec 6, 2023. Available from: https://reactome.org/PathwayBrowser.
[4] Brouwer, K. L., Keppler, D., Hoffmaster, K. A., et al. (2013). International Transporter Consortium. In vitro methods to support transporter evaluation in drug discovery and development. Clinical Pharmacology & Therapeutics, 94(1), 95-112.
[5] Sudsakorn S, Bahadduri P, Fretland J, Lu C. (2020). FDA Drug-drug Interaction Guidance: A Comparison Analysis and Action Plan by Pharmaceutical Industrial Scientists. Curr Drug Metab. 21(6):403-426.
[6] M12 DRUG INTERACTION STUDIES (Draft). 2022, Currently under public consultation. Available from: https://www.fda.gov/media/161199/download.
[7] Kotani, N., Maeda, K., Watanabe, T., et al. (2011). Culture Period-Dependent Changes in the Uptake of Transporter Substrates in Sandwich-Cultured Rat and Human Hepatocytes. Drug Metabolism and Disposition, 39(9), 1503–1510.
Related Services and Platforms




-

 In Vitro ADME ServicesLearn More
In Vitro ADME ServicesLearn More -

 DMPK BioanalysisLearn More
DMPK BioanalysisLearn More -

 Physicochemical Property StudyLearn More
Physicochemical Property StudyLearn More -

 Permeability and Transporter StudyLearn More
Permeability and Transporter StudyLearn More -

 Drug Distribution and Protein Binding StudiesLearn More
Drug Distribution and Protein Binding StudiesLearn More -

 Metabolic Stability StudyLearn More
Metabolic Stability StudyLearn More -

 Drug Interactions StudyLearn More
Drug Interactions StudyLearn More -

 Novel Drug Modalities BioanalysisLearn More
Novel Drug Modalities BioanalysisLearn More -

 Small Molecules BioanalysisLearn More
Small Molecules BioanalysisLearn More -

 Bioanalytical Instrument PlatformLearn More
Bioanalytical Instrument PlatformLearn More
Stay Connected
Keep up with the latest news and insights.