Plasma protein binding plays a central role in drug pharmacokinetics (PK) and pharmacodynamics (PD). Among these plasma proteins, the influence of Alpha-1 Acid Glycoprotein (AAG) on the distribution and clearance of drugs in the body is gaining increasing attention. Why is it so significant in PK, and how can researchers deepen their understanding of the importance of AAG protein binding in advancing new drug development?
The answers can be summarized as follows, and will be further discussed in the following sections:
Alpha-1 acid glycoprotein is a major plasma protein characterized by high affinity and low binding capacity. It affects drug bioavailability and distribution by binding to drugs, thereby influencing their efficacy and safety.
Multiple clinical cases indicate that drug binding to AAG can significantly impact drug pharmacokinetic properties.
When studying the effect of alpha-1 acid glycoprotein on PK, multiple experimental factors and a clear decision tree must be implemented.
What is Alpha-1 Acid Glycoprotein (AAG)
In plasma, albumin constitutes approximately 60%, far exceeding alpha-1 acid glycoprotein’s 1-3%. However, AAG still plays an indispensable role in drug binding and PK. It is a member of the acute-phase protein (APP) family, characterized as a highly acidic protein with a low isoelectric point. Synthesized in the liver, AAG circulates in healthy human plasma at concentrations of 0.5 to 1.0 mg/mL. Its levels can increase 2 to 6-fold in various disease states such as inflammation, infection, and cancer, which may affect drug efficacy. Therefore, monitoring alpha-1 acid glycoprotein levels is crucial for rational drug dose adjustment and for predicting and evaluating therapeutic outcomes. AAG exhibits high binding affinity for basic drugs and can also bind neutral and acidic drugs. Its biochemical properties enable it to bind a wide variety of drugs, and the diversity of its glycosylation adds complexity to drug interactions. This structural diversity makes AAG a plasma protein capable of interacting with numerous drugs, consequently influencing drug distribution, efficacy, and toxic side effects.
How AAG Protein Binding Influences Drug Pharmacokinetics
A comprehensive understanding of AAG's binding properties aids in predicting in vivo drug behavior, thereby improving the accuracy of pharmacokinetic/pharmacodynamic (PK/PD) models, optimizing drug dosing, enhancing efficacy, and reducing adverse reactions in early development stages. The following cases illustrate the clinical significance of studying drug-AAG binding.
Case 1: Explaining Species Difference
UCN-01 is a small-molecule protein kinase inhibitor primarily used for oncology indications. In clinical trials, UCN-01 displayed PK parameters inconsistent with animal studies. In animal models, it showed high clearance, high volume of distribution, and a moderate half-life. However, in humans, it demonstrated low clearance, low volume of distribution, and a long half-life.
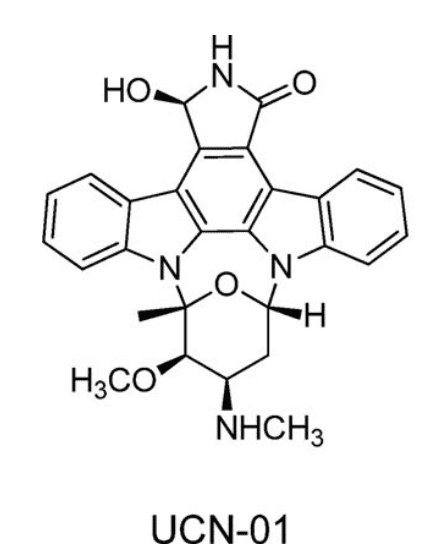
Figure 1. Chemical structure of UCN-01 [3]
Furthermore, the free fraction of UCN-01 in plasma decreased from 0.5-1.8% in preclinical species to 0.02% in humans. Further protein binding studies revealed a highly specific binding interaction between UCN-01 and human plasma alpha-1 acid glycoprotein. This specific binding affected the drug's free fraction, which varied with drug concentration, leading to nonlinear PK in the clinic. Concurrently, the Kd for human AAG and UCN-01 differed significantly from that in preclinical species, resulting in poor predictive accuracy using allometric scaling methods. UCN-01 has a very slow dissociation rate from human AAG, leading to a reduced volume of distribution and further impeding the interaction of the free drug with its target [1].
Case 2: Nonlinear PK and Dosing Regimen Optimization
Vismodegib is a drug for treating metastatic basal cell carcinoma. In Phase I clinical trials, it was expected to have high clearance and a short half-life, but low clearance and a long half-life were observed in humans. Additionally, the free fraction of Vismodegib differed between a single 150 mg dose (0.25%) and daily repeated 150 mg doses (0.65%). Research found that this difference partly originated from Vismodegib's higher affinity for human alpha-1 acid glycoprotein isoforms compared to rat isoforms. In vitro efficacy experiments showed a negative correlation between AAG concentration and target binding; supplementing the system with physiologically relevant AAG concentrations weakened the drug's effect. This indicates the need to fully consider the impact of plasma protein binding on the PK and PD of Vismodegib. In plasma samples from cancer patients, a high correlation was observed between alpha-1 acid glycoprotein and total Vismodegib concentrations at steady state, with total Vismodegib concentration correspondingly changing with AAG levels. Due to the nonlinear clinical PK, specific dosing regimens to maintain steady-state concentrations were evaluated in the Phase Ib trial. It was found that a once-daily 150 mg regimen ensured sufficient free Vismodegib to interact with the target for pharmacological effect after saturating AAG binding. This case highlights the importance of a deep understanding of drug-AAG protein binding to more accurately predict and adjust drug dosing [1].
Case 3: Nonlinear PK and Dosing Regimen Optimization
Imatinib is a tyrosine kinase inhibitor for chronic myeloid leukemia, exhibiting linear PK characteristics in patients. Studies show Imatinib's PK correlates with ABCB1 genotype, body weight, and alpha-1 acid glycoprotein levels.
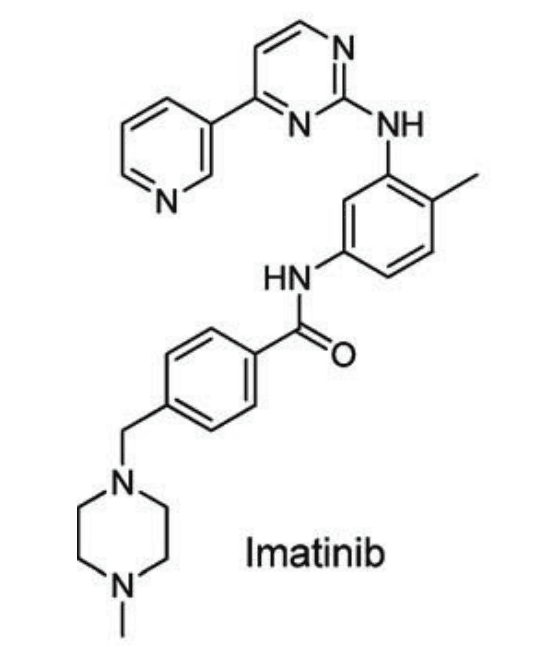
Figure 2. Chemical structure of Imatinib [3]
Although total plasma Imatinib concentrations show linear PK, its high affinity for AAG (approximately 55 times stronger than for albumin) results in a nonlinear relationship between the free fraction and the total plasma concentration. Notably, increased AAG levels have been associated with a weakened treatment response or the development of drug resistance in patients. It was also discovered that the plasticizer DEHP significantly impacts the free fraction of Imatinib in human plasma. In vitro, adding 800 μM di(2-ethylhexyl) phthalate (DEHP) to human plasma increased Imatinib's free fraction from 3.5% to 15.3% [1].
Case 4: Impact of Disease State on Protein Binding and PK
Alobresib is an anti-tumor BET protein inhibitor currently in clinical development, and its free fraction is substantially influenced by alpha-1 acid glycoprotein. When plasma AAG concentration increased from 0.9 mg/mL to 4.5 mg/mL, Alobresib's free fraction decreased more than 5-fold. In plasma from advanced cancer patients, Alobresib showed higher binding, with an average free fraction of 1.48%; whereas in plasma from healthy donors, the average free fraction was 5.49%. This difference may help explain the higher inter-individual variability in plasma PK observed in advanced cancer patients, particularly in those with higher AAG content, who exhibited very high plasma drug exposure [2].
Key Considerations for Studying Alpha-1 Acid Glycoprotein's Impact on Pharmacokinetics
The binding of a drug to AAG has important clinical significance when the following conditions are met [3]:
The drug has very high affinity for AAG, e.g., an association constant Ka > 105 M−1, indicating a strong tendency for the drug to exist mainly in a bound state in plasma.
The Volume of distribution at steady-state (Vdss) is small, suggesting limited distribution in the body, with most of the drug likely residing in the blood.
Alpha-1 acid glycoprotein is the primary binding protein for the drug in plasma, meaning the drug's distribution and kinetic properties may be influenced by AAG. During drug development, it is recommended to test drug binding not only to total plasma but also specifically to plasma albumin and AAG. This aids in the prospective prediction of human PK performance. For this purpose, experts in the field have developed a decision tree [1] (Figure 3).
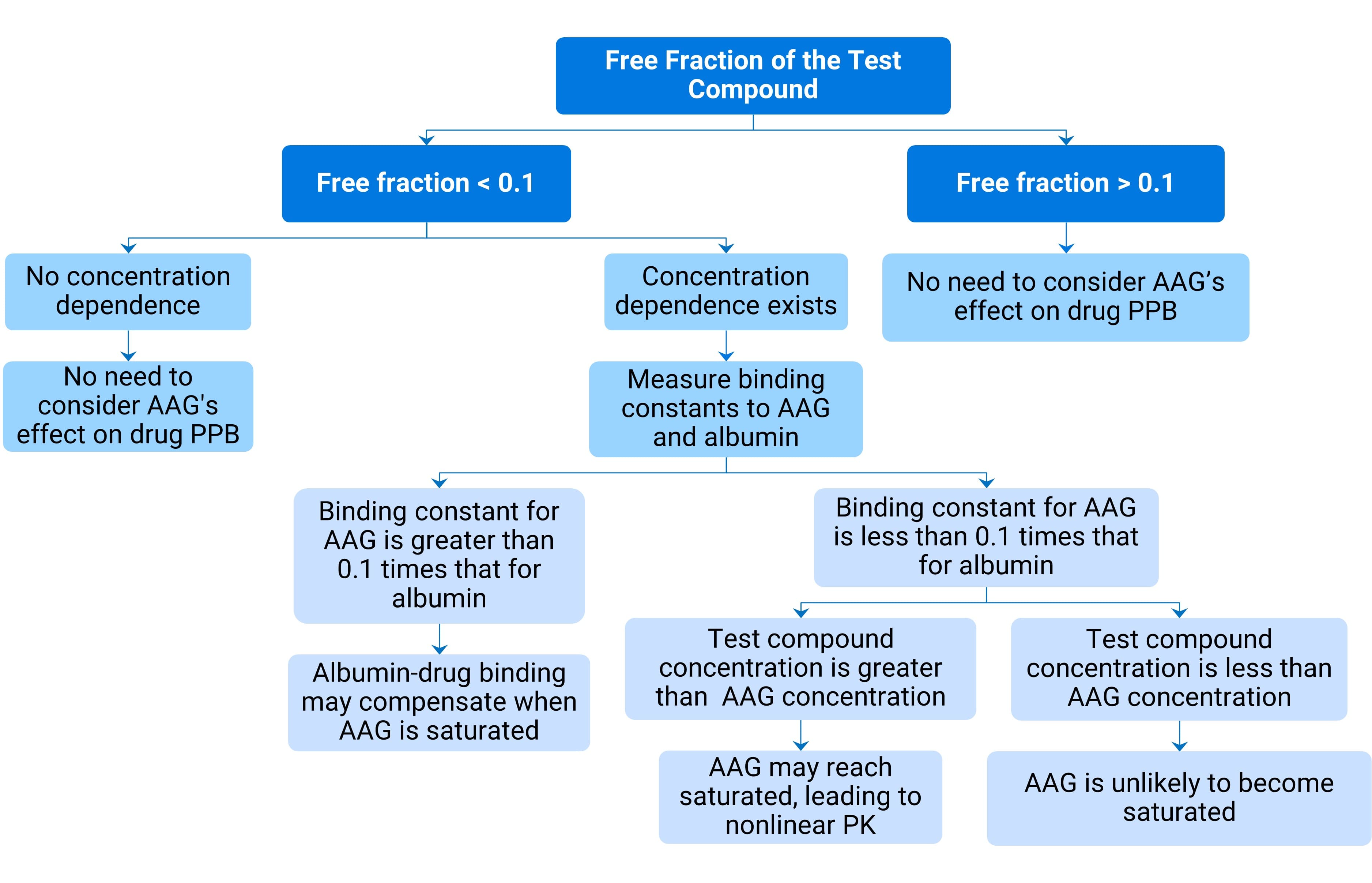
Figure 3. Decision tree for evaluating whether AAG constitutes a potential covariate leading to PK variability
When conducting AAG protein binding experiments, special attention should be paid to:
Consider the impact of plasticizers: Be aware of potential interference from plasticizers during matrix collection and storage. Some plasticizers, like DEHP, may leach from blood collection bags and affect drug-protein binding.
Concentration dependence of protein binding: Experimental design should account for the potential concentration dependence of drug binding to AAG.
Variability in AAG levels: AAG levels can change under different disease states, affecting drug binding. Therefore, experimental design should consider potential variations in AAG levels associated with disease.
Cross-species comparison: Particular attention is needed when drugs show significant binding differences across species.
By focusing on these aspects, researchers can more accurately design protein binding experiments and interpret results, thereby providing more reliable data for drug development and clinical application.
Concluding Remarks
In summary, alpha-1 acid glycoprotein is an important drug-binding plasma protein whose unique properties — particularly its variability during disease states — can significantly influence drug efficacy and toxicity. Therefore, it must be taken into account during drug development and clinical application. Furthermore, AAG also exhibits potential as a biomarker for reflecting disease status and response to drug therapy. To fully incorporate these considerations, the accurate measurement and analysis of AAG-drug binding are essential.
Authors: Mengying Li, Jie Wang, Xiangling Wang, Genfu Chen
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Smith SA, Waters NJ. Pharmacokinetic and Pharmacodynamic Considerations for Drugs Binding to Alpha-1-Acid Glycoprotein. Pharm Res. 2018 Dec 28;36(2):30
[2] Kelly Wang, Bernard Murray. The influence of alpha-1 acid glycoprotein binding on total plasma free fraction and the effect on clinical pharmacokinetics. Poster presented at the 26th North American ISSX/JSSX meeting, September 15-18, 2024.
[3] Bohnert T, Gan LS. Plasma protein binding: from discovery to development. J Pharm Sci. 2013 Sep;102(9):2953-94.
Stay Connected
Keep up with the latest news and insights.













