Since the 1960s, glucagon-like peptide-1 (GLP-1) has garnered significant attention due to its extensive physiological effects in the human body. The development of peptide drugs targeting GLP-1 has shown remarkable progress, not only with single-target receptor agonists (e.g., semaglutide) but also with multi-target agonists, such as the dual GLP-1 and GIP receptor agonist tirzepatide, whose clinical efficacy in the field of obesity has been widely noted [1]. Currently, several triple-target receptor agonists have entered clinical trials.
GLP-1 receptor agonists can be categorized into three classes: GLP-1 short peptide mutants, e.g., beinaglutide; chemically modified GLP-1 receptor agonists, e.g., liraglutide, semaglutide, polyethylene glycol loxenatide, etc.; and GLP-1 receptor agonists conjugated or fused with proteins (antibodies, Fc fragments, albumin, etc.). [2] Current marketed GLP-1 analogs predominantly incorporate fatty acid chain modifications to enhance pharmacokinetic properties. For such lipid chain-modified GLP-1 peptide drugs, comprehensive absorption, distribution, metabolism, and excretion (ADME) studies are essential to elucidate their in vivo behavior. Radiolabeling techniques serve as a robust platform for ADME investigations of GLP-1 analogs. This article will focus on the radiolabeling synthesis strategies and key ADME study considerations for lipid chain-modified GLP-1 analogs.
Radiolabeling Synthesis of GLP-1 Peptide Drugs
The radiolabeling synthesis of GLP-1 peptide drugs necessitates a comprehensive consideration of several key factors, including administered dose, radionuclide, labeling site, number of labels, and synthetic route.
Considerations for Labeling Sites and Number of Labels
The selection of a radionuclide can refer to the article: Decoding DMPK Profiles of 37 FDA-Approved Peptides: Insights and Radiolabeling Considerations. For GLP-1 peptide drugs, 14C is a highly recommended radiolabeling nuclide.
Choosing the location for radiolabeling is crucial. The introduced radiolabeled compound should be able to trace important biotransformation pathways or circulating metabolites. Currently, the preferred labeling sites for lipid chain-modified GLP-1 analogs are the lipid moiety and the fragment connecting it to the amino acid [6-8].
The molecular weight of GLP-1 peptide drugs is generally between 3,000 and 6,000 Da, with low in vivo dosing. The administered doses for marketed GLP-1 peptide drugs in preclinical animals range from 0.15 to 3 mg/kg. If labeling only one carbon atom (specific activity ~45-55 mCi/mmol), it is usually insufficient to meet the quantitative requirements for mass balance and radioactive metabolite profiling studies. Therefore, a strategy of labeling multiple carbon atoms is often adopted. For example, tirzepatide[6] is labeled with four carbon atoms.
Case Study: Multi-Site Labeling of a GLP-1 Peptide Drug
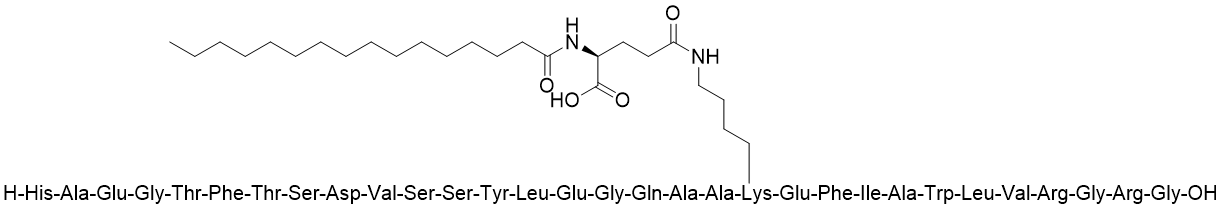
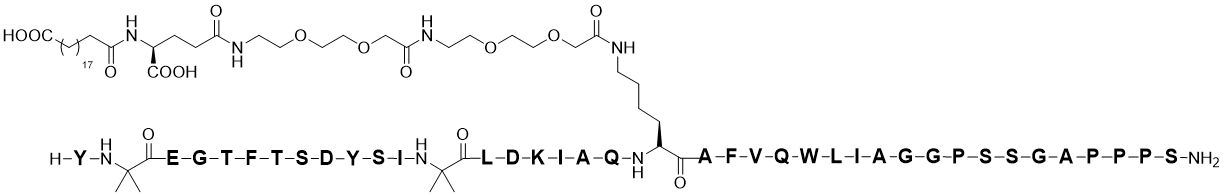
Tirzepatide has a molecular weight of 4,814 Da. Researchers introduced four 14C radiolabeled carbon atoms into the side chain of tirzepatide[6] (Figure 1), achieving a radioactive specific activity of 208 mCi/mmol (43 µCi/mg). This met the requirements for subsequent ADME studies (SD rats: 3 mg/133 µCi/kg; cynomolgus monkeys: 0.5 mg/20 µCi/kg; humans: 4.1 mg/100 µCi/person).
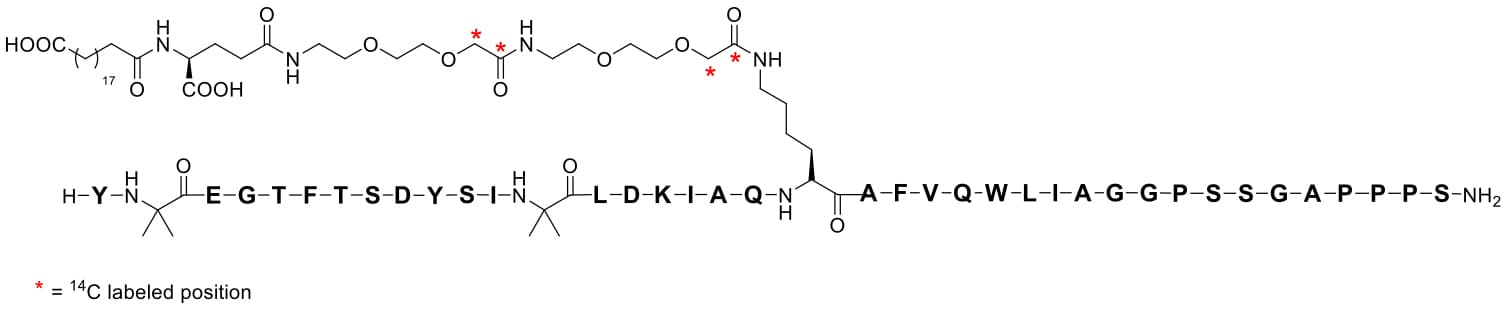
Figure 1. The chemical structure of 14C-labeled tirzepatide
Tirzepatide contains a C20 fatty diacid portion. The fatty diacid portion (icosanedioic acid) is connected to the lysine residue side chain via glutamic acid and two (2-(2-aminoethoxy)ethoxy)acetic acid units (AEEA). The synthesis of [14C]-tirzepatide can start from a tirzepatide resin intermediate, using solid-phase peptide synthesis (SPPS) technology to sequentially connect the double-labeled [14C]AEEAc (note: connected twice), Fmoc-Glu-OtBu (fluorenylmethyloxycarbonyl-L-glutamic acid 1-tert-butyl ester), and C20 DA-OtBu (eicosanedioic acid mono-tert-butyl ester) to the peptide backbone. Finally, the peptide chain is cleaved from the resin and purified to obtain [14C]-tirzepatide.
According to the industry white paper on peptide drug ADME studies released in 2023 [9], if a peptide drug contains novel non-natural amino acids and/or is embedded with any new organic linkers not present in marketed drugs, it is generally recommended to radiolabel the new moiety to conduct radio ADME studies, thereby characterizing its metabolic and elimination pathways.
GLP-1 peptide drugs can be classified into two categories based on their structural features: linear peptides and cyclic peptides.
For Radiolabeling of GLP-1 linear peptide, 14C can be introduced into modified fatty acid side chains, such as 2-(2-aminoethoxy) acetic acid or the terminal fatty acid. This method enables the labeling of 4 or more 14C atoms, achieving a radioactive specific activity of up to 200 mCi/mmol.
For radiolabeling of GLP-1 cyclic peptide, 14C can be incorporated into modified small cyclic peptide fragments. For example, introducing 2 or 4 14C atoms via fully labeled glycine into the cyclic peptide structure allows sufficient radioactive specific activity to be attained.
WuXi AppTec DMPK has successfully developed various 14C-labeled small molecule building blocks, including key compounds for amino acids and the fatty acid-modified side chains of GLP-1 peptide drugs, such as 14C-labeled C20 fatty acid and 14C-labeled 2-(2-aminoethoxy)acetic acid.
Considerations for Radiolabeled ADME Studies of GLP-1 Peptide Drugs
As of the end of 2024, the FDA and EMA have approved a total of 9 GLP-1 peptide drugs (Table 3), while the NMPA has approved 2 GLP-1 peptide drugs (polyethylene glycol loxenatide and beinaglutide) [1]. By summarizing the ADME study contents of the 9 GLP-1 peptide drugs approved by the FDA and EMA, the ADME research considerations for GLP-1 peptide drugs are categorized as follows.
Mass Balance
Except exenatide (the first GLP-1 drug marketed in 2005) and the fusion protein drugs dulaglutide and albiglutide, all currently FDA- and EMA-approved GLP-1 analogs have utilized radiolabeled isotopes for mass balance studies.
The homology between the rat and human GLP-1 receptor (GLP-1R) is 90%, while the homology between the monkey and human GLP-1 receptor is even closer, reaching 99% [10]. Therefore, in the mass balance studies of marketed GLP-1 class drugs (e.g., liraglutide, semaglutide, and tirzepatide), both rats and monkeys were used for preclinical mass balance studies, and human mass balance studies were conducted in parallel [6-8]. Compared to the structure of exenatide, lixisenatide differs only by the absence of one proline at the carboxyl terminus and the addition of six lysines (Table 3), which enhances its stability in vivo. As the peptide chains of both are composed of natural amino acids, comprehensive radiolabeled ADME studies were not conducted. For lixisenatide, only a milk excretion study was performed using lactating female rats [11].
Based on preclinical data from rats and monkeys in the literature [6,7], renal excretion is the primary excretion pathway for fatty acid-modified GLP-1 peptide drugs (accounting for 40% to 80% of the administered dose), while fecal excretion accounts for 10% to 50%. Notably, however, structural modification strategies (such as fatty acid side chain modification) can alter the excretion pathways.
Researchers conducted radiolabeled ADME studies of [14C]-tirzepatide[6,7] in rats, monkeys, and humans, achieving mass balance recovery rates above 90% (Table 1). The results showed that the excretion proportion of tirzepatide in urine was comparable to that in feces in rats and monkeys, while in humans, the urinary excretion proportion was slightly greater than fecal excretion. In contrast, lixisenatide and exenatide are primarily cleared via glomerular filtration[11,12].
Table 1. Summary of radioactive excretion after a single subcutaneous dose of [14C]-tirzepatide
Parameter | Human (%Dose) n=6 | SD Rat (%Dose) n=4 | Cynomolgus Monkey (%Dose) n=4 |
Dose | ~4.1 mg/person | 3 mg/kg | 0.5 mg/kg |
100 µCi/person | 133 µCi/kg | 20 µCi/kg | |
Collection Period | 0-36 days | 0-14 days | 0-28 days |
Gender | Male | Male | Male |
Urine | 66.1 ± 3.6 # | 45.0 ± 2.3 | 49.4 ± 4.6 |
Feces | 33.2 ± 4.2 # | 47.1 ± 0.8 | 35.0 ± 2.5 |
Other* | NA | 19.6 ± 2.9 | 12.8 ± 3.4 |
Total Recovery | 99.4 ± 4.0 # | 112.0 ± 2.0 | 97.2 ± 1.8 |
*: Includes animal carcass, exhaled air, cage wash, etc.; #: Data obtained using extrapolation.
Long-acting GLP-1 drugs have long clearance half-lives; for example, the clearance half-life of semaglutide in humans is approximately one week. Therefore, mass balance experiments require longer collection times, necessitating adjustments to sample collection intervals (e.g., from 24 h to 48-72 h) and timely modification of the sample collection scheme based on measurement results.
Distribution
Currently, many marketed GLP-1 drugs as well as investigational GLP-1 drugs commonly employ quantitative whole-body autoradiography (QWBA) for tissue distribution studies in preclinical animals [6-8]. Applying QWBA to study total radioactivity distribution throughout the body provides complete and detailed tissue distribution results. Based on the exposure levels and elimination half-lives in various tissues, it allows for assessing acceptable radioactive safety doses in humans. For example, after a single subcutaneous injection of a 14C-labeled GLP-1 peptide drug in rats, its distribution profile at 24 hours post-dose was studied using QWBA (Figure 2). The profile shows extremely high distribution concentration in the kidneys, followed by the liver and small intestine contents. Medium concentrations are observed in the lungs and brown fat, while low concentrations are seen in the brain, stomach contents, and bone marrow. This indicates that at 24 hours post-dose, the drug is mainly distributed in the kidneys, liver, and small intestine contents.
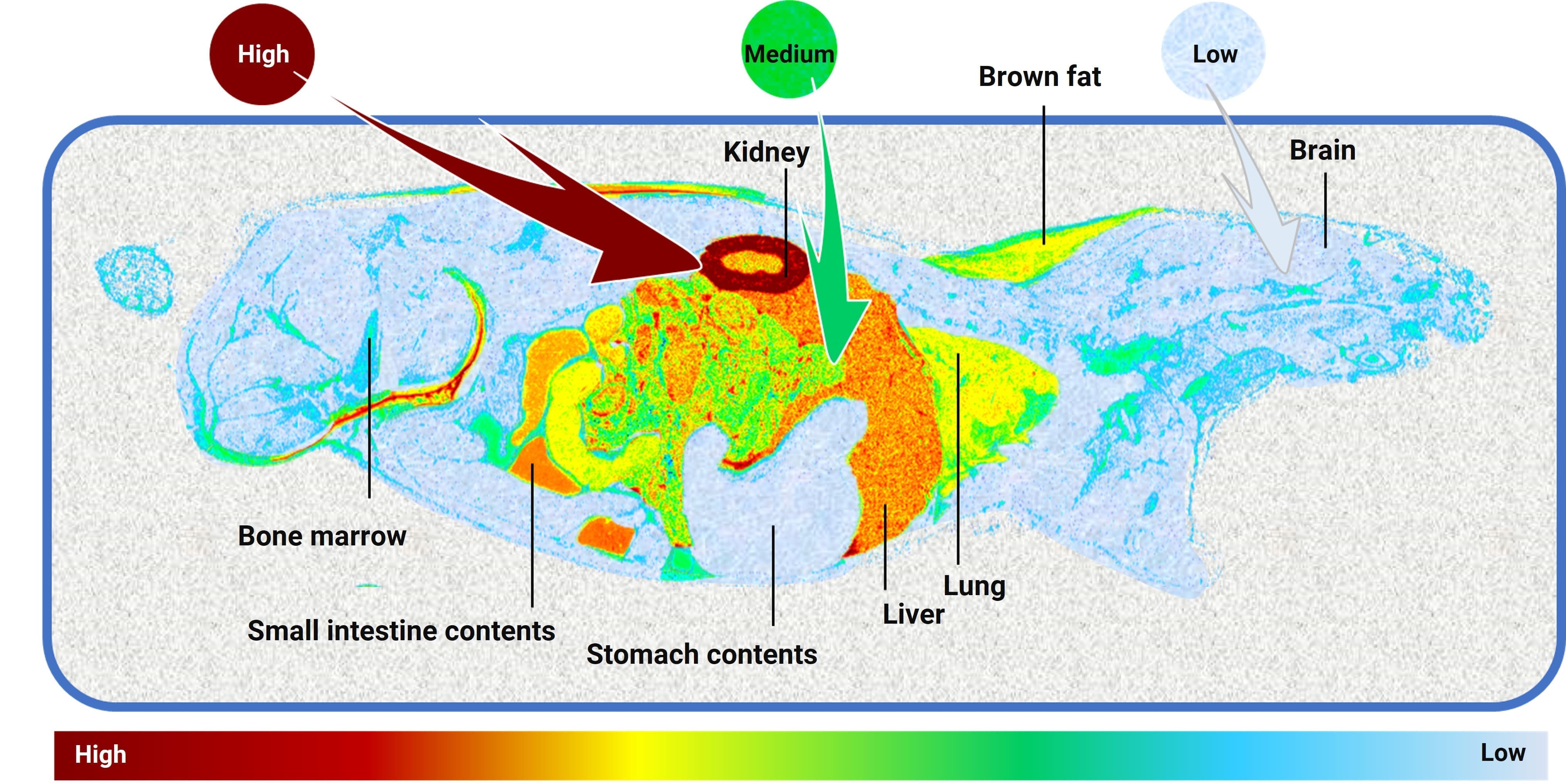
Figure 2. QWBA profile at 24 hours after a single subcutaneous injection of a 14C-labeled GLP-1 peptide in rats
Metabolism
The metabolic profile of GLP-1 peptide drugs is a crucial aspect of their ADME studies. Understanding metabolism is essential for assessing drug safety and efficacy. Liraglutide, semaglutide, and tirzepatide have undergone metabolic studies using radiotracer technology, including in vitro hepatocyte and plasma metabolism studies, and metabolite identification studies in feces, urine, and plasma from rats, monkeys, and humans [6-8].
Depending on their structural modifications, GLP-1 analogs may undergo different primary metabolic pathways. For instance, fatty acid-modified drugs like semaglutide and tirzepatide primarily undergo hydrolysis of the peptide backbone and β-oxidation of the fatty acid [6,7]. In contrast, GLP-1 analogs without fatty acid modification, e.g., lixisenatide, primarily undergo hydrolysis of the peptide backbone [11].
It is noteworthy that the metabolite profiles of fatty acid-modified GLP-1 peptide drugs can vary significantly across different matrices. For example, the main component of [14C]-tirzepatide in rat, monkey, and human plasma is the parent drug, with minor metabolites arising from hydrolysis of the peptide backbone while the fatty acid side chain remains intact. However, in excretion matrices (urine and feces), the parent drug is not detected. The main metabolites result from hydrolysis of the peptide backbone and β-oxidation of the fatty acid side chain [6]. The β-oxidation of the fatty acid side chain leads to significant changes in the hydrophilicity of the metabolite molecules in excretion matrices, making their retention on conventional liquid chromatography columns challenging and posing difficulties for metabolite identification.
Pharmacokinetics (PK)
Except for oral semaglutide, the currently marketed GLP-1 peptide drugs are all administered via subcutaneous injection, characterized by a long half-life (approximately one week). PK data may vary significantly between small and large animals. For example, after a single subcutaneous dose of [14C]-tirzepatide, the Cmax/Tmax/t1/2 in cynomolgus monkey plasma was about 0.3/3/4 times that in SD rat plasma [6]. The Tmax times for tirzepatide and [14C]-tirzepatide in plasma were essentially the same. Notably, in rat plasma, the mean systemic exposure of tirzepatide accounted for about 87% of the total plasma radioactive exposure, while in monkeys it was 84%, indicating that the main component in rat and monkey plasma is the parent drug.
Table 2. Summary of PK parameters after a single subcutaneous dose of tirzepatide or [14C]-tirzepatide
PK Parameter | SD Rat | Cynomolgus Monkey | ||||
[¹⁴C] Whole Blood | [¹⁴C] Plasma | TZP Plasma | [¹⁴C] Whole Blood | [¹⁴C] Plasma | TZP Plasma | |
Cmax (ng eq/g or ng/mL) | 8,340 | 16,300 | 19,600 | 2,830 | 5,160 | 6,410 |
Tmax (hours) | 6 | 6 | 6 | 5 | 18 | 15 |
t1/2 (hours) | 57.2 | 25.6 | 9.03 | 101 | 103 | 56.3 |
AUC0-inf (ng eq·h/g or ng·h/mL) | 285,000 | 551,000 | 478,000 | 381,000 | 654,000 | 549,000 |
[14C] = [14C]-tirzepatide; TZP = tirzepatide.
Final Perspectives
Radiolabeling provides a powerful tool for the ADME studies of GLP-1 peptide drugs. Future research on GLP-1 analogs will likely focus on the development of oral formulations and the exploration of novel drug delivery systems to further improve patient compliance and therapeutic efficacy. WuXi AppTec DMPK Department possesses over a decade of experience in radiolabeled project research and hundreds of submission experiences for radiolabeled test articles to the FDA and NMPA. With well-established technology platforms for radiolabeled compound synthesis, mass balance, tissue distribution, metabolite profiling, and metabolite identification, we empower clients to complete numerous project submissions and accelerate the global drug development process.
Table 3. Summary of GLP-1 Peptide Drugs Approved by FDA and EMA
No. | Trade Name | Active Ingredient | Developer / First Approval | Molecular Weight (Da) | Peptide Structure |
1 | Byetta | Exenatide | AstraZeneca / 2005 | 4,187 | H-His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-SerNH₂ |
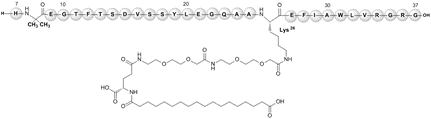
2 | Victoza | Liraglutide | Novo Nordisk / 2010 | 3,751 |
|
3 | Lyxumia | Lixisenatide | Sanofi, Zealand Pharma / 2013 | 4,859 | H-His-Gly-E-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Ser-Lys-Lys-Lys-Lys-Lys-Lys-NH₂ |
4 | Trulicity | Dulaglutide | Eli Lilly and Company / 2014 | ~63,000 | H-His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Glu-Gln-Ala-Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Lys-Gly-Gly-Gly-OH (Fc fusion protein) |
5 | Tanzeum | Albiglutide | GlaxoSmithKline LLC / 2014 | ~73,000 | H-His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Lys-Gly-Arg-NH₂ (Albumin fusion protein) |
6 | Ozempic | Semaglutide | Novo Nordisk Inc / 2017 | 4,114 |
|
7 | Mounjaro | Tirzepatide | Eli Lilly and Company / 2022 | 4,814 |
|
8 | Rybelsus | Oral Semaglutide | Novo Nordisk / 2019 | 4,114 | NA |
9 | Bydureon | Exenatide extended-release | AstraZeneca / 2012 | 4,187 | NA |
Authors: Ya Ding, Dongdong Xiong, Jianglin Wu, Lian Guo, Lingling Zhang
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Gong B, Yao Z, Zhou C, et al. Glucagon-like peptide-1 analogs: Miracle drugs are blooming?[J]. European Journal of Medicinal Chemistry, 2024: 116342.
[2] Technical Guideline for Pharmaceutical Research and Evaluation of Recombinant Glucagon-Like Peptide-1 Receptor Agonists (for Public Comment), 2024-12-16.
[3] Knudsen LB, Lau J. The Discovery and Development of Liraglutide and Semaglutide. Front Endocrinol (Lausanne). 2019; 10:155. Published 2019 Apr 12. doi:10.3389/fendo.2019.00155.
[4] Davide Audisio, Victor Babin, Frédéric Taran. Late-Stage Carbon-14 Labeling and isotope exchange: emerging opportunities and future challenges. JACS Au, 2022, 2: 1234-1251.
[5] Elmore CS. The use of isotopically labeled compounds in drug discovery [J]. Annu Rep Med Chem, 2009, 44: 515-534.
[6] Martin, J. A.; Czeskis, B.; Urva, S., et al. Absorption, distribution, metabolism, and excretion of tirzepatide in humans, rats, and monkeys[J]. Eur J Pharm Sci. 2024, 202: 106895.
[7] Jensen, L., Helleberg, H., Roffel, A., van Lier, J.J., Bjørnsdottir, I., Pedersen, P.J., et al.,2017. Absorption, metabolism, and excretion of the GLP-1 analogue semaglutide in humans and nonclinical species. Eur. J. Pharm. Sci. 104, 31–41.
[8] US Food and Drug Administration. Pharmacology Review(s) (PDF) http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022341s000pharmr_P1.pdf (2008).
[9] He M M, Zhu S X, Cannon J R, et al. Metabolism and excretion of therapeutic peptides: current industry practices, perspectives, and recommendations[J]. Drug Metabolism and Disposition, 2023, 51(11): 1436-1450.
[10] European Medicines Agency. Assessment report. http://www.ema.europa.eu/en/medicines/human/EPAR/ozempic (2017).
[11] US Food and Drug Administration. Pharmacology Review(s) (PDF) http://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208471Orig1s000PharmR.pdf (2016).
[12] US Food and Drug Administration. BYETTA™(exenatide) Label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021773lbl.pdf (2005).
Related Services and Platforms




-

 Radiolabeled In Vivo ADME StudyLearn More
Radiolabeled In Vivo ADME StudyLearn More -

 Novel Drug Modalities DMPK Enabling PlatformsLearn More
Novel Drug Modalities DMPK Enabling PlatformsLearn More -

 Radiolabeled MetID (Metabolite Profiling and Identification)Learn More
Radiolabeled MetID (Metabolite Profiling and Identification)Learn More -

 Radiolabeled Non-Clinical In Vivo ADME StudyLearn More
Radiolabeled Non-Clinical In Vivo ADME StudyLearn More -

 Quantitative Whole-body Autoradiography (QWBA)Learn More
Quantitative Whole-body Autoradiography (QWBA)Learn More -

 Human Radiolabeled Mass Balance StudyLearn More
Human Radiolabeled Mass Balance StudyLearn More -

 Radiolabeled Compound SynthesisLearn More
Radiolabeled Compound SynthesisLearn More -

 PROTAC DMPK ServicesLearn More
PROTAC DMPK ServicesLearn More -

 ADC DMPK ServicesLearn More
ADC DMPK ServicesLearn More -

 Oligo DMPK ServicesLearn More
Oligo DMPK ServicesLearn More -

 PDC DMPK ServicesLearn More
PDC DMPK ServicesLearn More -

 Peptide DMPK ServicesLearn More
Peptide DMPK ServicesLearn More -

 mRNA DMPK ServicesLearn More
mRNA DMPK ServicesLearn More -

 Covalent Drugs DMPK ServicesLearn More
Covalent Drugs DMPK ServicesLearn More
Stay Connected
Keep up with the latest news and insights.