Unlike small molecules or single biological macromolecules, antibody–oligonucleotide conjugates (AOCs) are ternary constructs of antibody, oligonucleotide, and linker. The physicochemical properties of each component and their interactions can profoundly influence pharmacology and ADME. A deep understanding of AOC structural features and their ADME effects is therefore valuable: it informs in vitro and in vivo metabolite-identification studies, PK study design, and exposure-response analyses, and it guides molecular design and conjugation strategy — for example, choosing stable versus cleavable linkers, optimizing conjugation sites and the oligonucleotide–to–antibody ratio (OAR), and refining oligonucleotide chemistries. These insights help balance in vivo stability, targeted delivery, and intracellular release to improve efficacy, reduce off-target toxicity, and support safety assessment and clinical translation. This article will outline the overall structure of AOC drugs and focus on discussing the structure and mechanism of action of the antibody and oligonucleotide components. The linker, conjugation methods, and metabolite identification of AOC drugs will be elaborated in later articles.
What Are Antibody–Oligonucleotide Conjugates (AOCs) and Their Development
Antibody–oligonucleotide conjugates (AOCs) are biotherapeutics in which antibodies and oligonucleotides are covalently linked using specific conjugation technologies. By combining the antibody’s targeted delivery capabilities with the sequence-specific activity of the oligonucleotide payload, AOCs can deliver therapeutic oligonucleotides to defined cells or tissues, effectively addressing the extrahepatic delivery challenges faced by conventional oligonucleotide drugs and offering new therapeutic strategies for genetic disorders, cancer, and other hard-to-treat diseases [1]. The research and clinical development of AOCs have progressed rapidly. Representative companies such as Avidity Biosciences reported clinical data for their candidate AOC 1001 in October 2023 (NCT05027269), showing an average reduction of approximately 45% in the levels of the disease-causing protein DMPK (dystrophia myotonica-protein kinase) in patient muscle. AOC 1001 became the first AOC to enter global Phase III trials in Q2 2024. Other companies, including Dyne Therapeutics, Tallac Therapeutics, and Denali Therapeutics, are also advancing AOC pipelines addressing muscle diseases, central nervous system disorders, and tumors (Table 1).
Table 1. Summary of investigational drug candidates from representative companies [2]
Drug Name | Company | Antibody Type | Drug Payload | Action Target | Target Tissue | Indication | Research Phase | Latest Clinical Results |
AOC 1001 | Avidity | TfR1-targeting mAb | DMPK siRNA | DMPK | Muscle tissue | DM1 | Phase III | Treated participants showed an average 45% reduction in DMPK. |
AOC 1044 | Avidity | TfR1-targeting mAb | Exon-44-skipping PMO | Exon 44 | Muscle tissue | DMD | Phase III | Significant increase in dystrophin protein. |
AOC 1020 | Avidity | TfR1-targeting mAb | DUX4 siRNA | DUX4 | Muscle tissue | FSHD | Phase III | Decrease in cDUX biomarker. |
DYNE-101 | Dyne | TfR1-targeting Fab | DMPK ASO | DMPK | Muscle tissue | DM1 | Phase I/II | Improvement in patient myotonia symptoms. |
DYNE-251 | Dyne | TfR1-targeting Fab | Exon-51-skipping PMO | Exon 51 | Muscle tissue | DMD | Phase I/II | Increase in patient average dystrophin levels. |
DYNE-302 | Dyne | TfR1-targeting Fab | DUX4 ASO | DUX4 | Muscle tissue | FSHD | Preclinical | None |
Undisclosed | Denali [3] | TfR1-targeting mAb via Fc engineering | Undisclosed ASO | Undisclosed | Brain | Undisclosed | Preclinical | None |
Undisclosed | Ionis | CD29-targeting mAb | Undisclosed siRNA | Undisclosed | Undisclosed | Undisclosed | Preclinical | None |
Tac-001 | Tallac | CD22-targeting mAb | CpG (TLR9 agonist) | TLR9 | B cells | Advanced solid tumors | Phase I/II | Stable Disease (SD) in some patients. |
ALTA-002 | Tallac | SIRPα-targeting mAb | CpG (TLR9 agonist) | TLR9 | Dendritic cells | Solid tumors | Preclinical | None |
Note: TfR1: transferrin receptor 1; CD29: integrin beta-1; CD22: Siglec-2; DMPK: dystrophia myotonica protein kinase; siRNA: small interfering RNA; PMO: phosphorodiamidate morpholino oligomer; DUX4: double homeobox protein 4; ASO: antisense oligonucleotide; CpG: cytosine phosphoric acid guanine; TLR9: toll like receptor; DM1: myotonic dystrophy type 1; DMD: duchenne muscular dystrophy; FSHD: facioscapulohumeral muscular dystrophy.
Structural Features of Antibody–Oligonucleotide Conjugates (AOCs)
AOC therapeutics are primarily composed of three parts: antibody, linker, and oligonucleotide — assembled by various conjugation strategies. The interplay among these components determines targeting efficiency, payload-release kinetics, and ultimately therapeutic efficacy. The mechanism of action involves the following key steps:
Target recognition: the antibody moiety specifically recognizes and binds antigens on the target cell surface.
Internalization and trafficking: the antigen-antibody complex is internalized via endocytosis to form endosomes, which subsequently fuse with lysosomes.
Payload release: cleavable linkers release the payload upon cleavage, whereas non-cleavable linkers rely on degradation of the antibody backbone to liberate the active payload. The released active oligonucleotide (either free or still conjugated to linker fragments) must then escape from the lysosome compartment into the cytosol to reach its site of action.
Gene regulation: siRNA is loaded into the RNA-induced silencing complex (RISC) and mediates target mRNA cleavage via Ago2, affecting gene silencing. ASO regulates genes by several mechanisms, including steric blockade of translation through hybridization to mRNA, binding to pre-mRNA to modulate alternative splicing (e.g., inducing exon skipping or promoting exon inclusion), or recruitment of RNase H to degrade target RNA.
In summary, targeted delivery, internalization and trafficking, lysosomal release and escape of the oligonucleotide, and intracellular gene regulation form a tightly regulated network in which all three components are critical. This article will focus on the structure–function relationships of the antibody and oligonucleotide components.
Antibody Design and Selection in Antibody–Oligonucleotide Conjugates (AOCs)
Antibodies primarily serve as targeting moieties for delivery. Common antibody formats include monoclonal antibodies (mAbs), polyclonal antibodies (pAbs), recombinant antibodies, nanobodies, bispecific antibodies (BsAbs), and antibody fragments. mAbs are the most commonly used antibody type in AOC applications, typically employing immunoglobulin G (IgG) as the basic scaffold because of its favorable homogeneity. For mAbs, site-specific engineering and conjugation strategies directed at the intact antibody or particular domains (such as the fragment antigen-binding (Fab) or fragment crystallizable (Fc) regions) have been widely adopted by industry. The following sections discuss antibody structure as well as antibody selection and design considerations.
Antibody structure
An IgG-type antibody has a Y-shaped architecture composed of two identical heavy chains and two identical light chains. The two arms of the Y form the Fab regions, each containing the antigen-binding site made up of three complementarity-determining regions (CDRs) from the heavy chain and three from the light chain; these sites are responsible for the specific recognition of antigen epitopes. The stem of the Y is the Fc region, a constant region that determines antibody isotype and mediates effector functions such as Fc receptor binding, complement activation, and FcRn-mediated recycling that prolongs serum half-life [4]. The detailed structural and functional sites are shown in Figure 1.
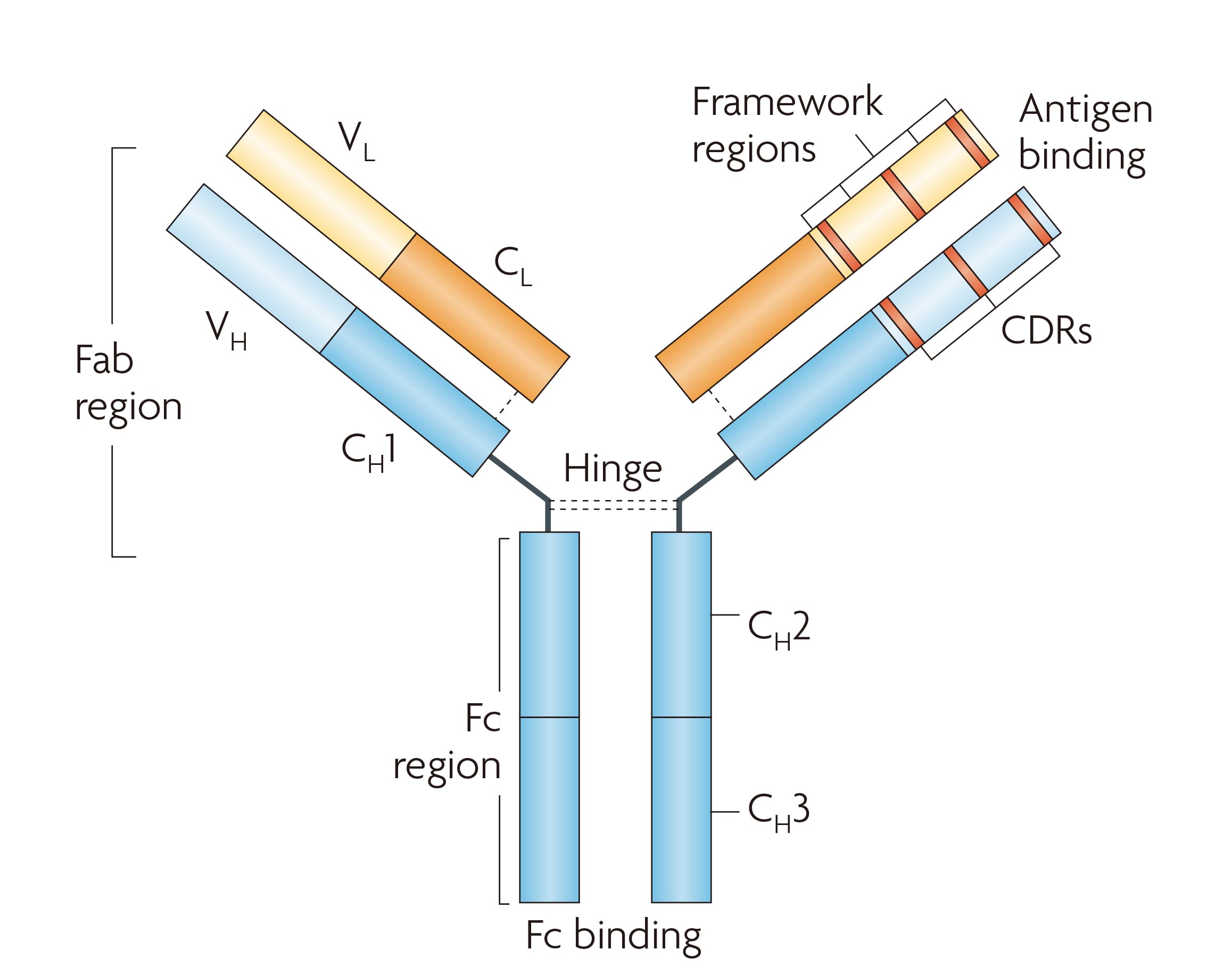
Figure 1. Structure of the antibody [5]
Antibody selection and design
AOCs in development employ a variety of antibodies against different targets, including anti–TfR1, anti–CD29, anti–CD22, anti–SIRPα, and anti–EGFR [6]. Antibody selection and design are guided by multiple considerations, such as: the expression level and accessibility of the corresponding receptor/antigen on target tissue cells; whether antibody binding can trigger endocytosis; off-target distribution in non-target tissues; the types of residues available for site-specific conjugation; and whether Fc functions should be retained or attenuated to modulate half-life and immune effector activities.
As shown in Table 1 and Figure 2, for muscle-targeting anti-TfR1 antibodies, Avidity employs a full-length monoclonal IgG scaffold, and the Fc region’s Fc–FcRn recycling substantially extends plasma half-life, enabling sustained exposure and longer dosing intervals. By contrast, Dyne uses a Fab fragment: its smaller size improves tissue penetration and, lacking an Fc, it avoids Fc-mediated effector functions, which helps the drug penetrate muscle tissue and reduces immune-related risks; however, it has a shorter half-life and may require more frequent dosing or additional modifications. The central trade-off is balancing enhanced tissue penetration against maintaining systemic exposure duration, while deciding whether to retain or remove Fc-mediated functions (e.g., FcRn recycling to extend half-life, or FcγR-mediated antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity) and the attendant implications for immunological safety. Unlike the strategies used by Avidity and Dyne, Denali’s anti-TfR1 antibody is engineered to enable central nervous system (CNS) delivery: Fc modifications enhance interaction with transferrin receptor 1 (TfR1) on the blood–brain barrier, promoting receptor-mediated endocytosis and transcytosis to transport the antibody and its oligonucleotide cargo into the brain, thereby providing a viable route for treating CNS disorders [7].
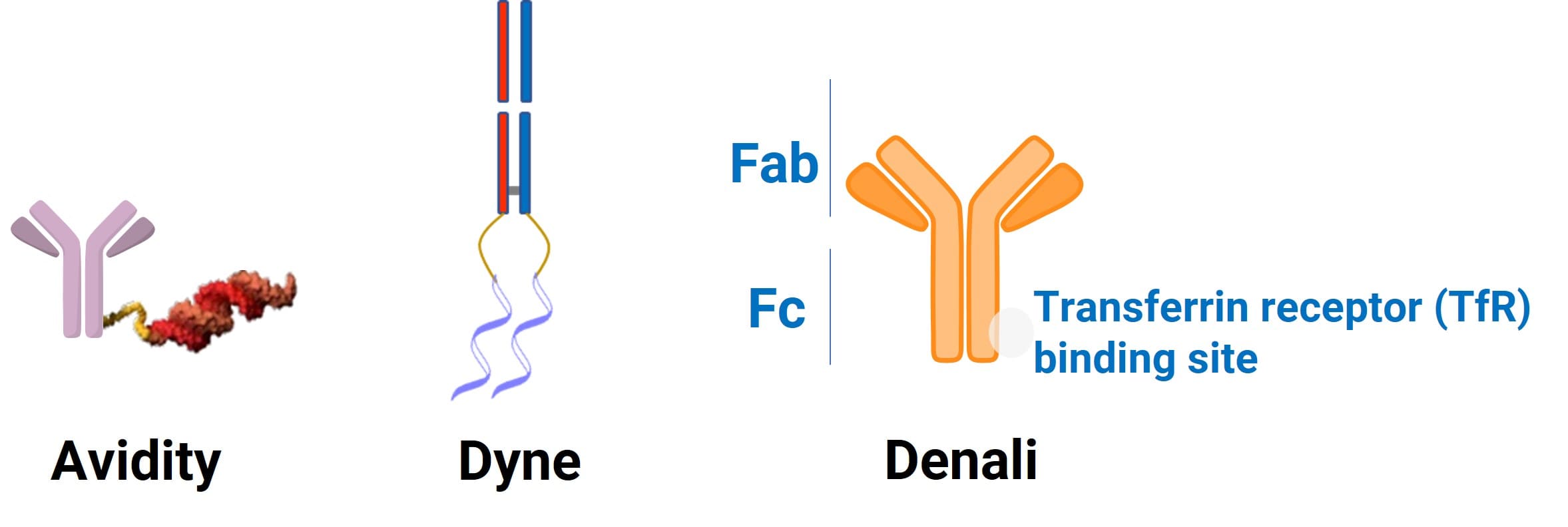
Figure 2. Comparison of antibody designs from three companies.
In an Ionis patent [8], AOCs were prepared in both full-length IgG and Fab formats of anti-CD29. The Fc region of a full-length IgG normally carries two N-linked glycans, and conjugation at these N-glycan sites typically yields a mixture of species with OAR = 1 and OAR = 2 (with some unmodified material at OAR = 0). The patent reports producing such mixed-OAR AOCs with an approximate 1:1 ratio of OAR1 to OAR2. For the Fab format, a specific strategy was employed to generate AOCs with a single oligonucleotide per antibody (OAR = 1), as illustrated in Figure 3. Studies have shown [9] that the number of oligonucleotides attached to an antibody markedly affects AOC pharmacokinetics and efficacy: high OAR can increase molecular heterogeneity, alter hydrophilicity/hydrophobicity, and exposure of Fc functions, thereby accelerating clearance and reducing effective exposure and activity. Thus, optimizing AOCs requires balancing OAR, conjugation site, linker design, and stability against in vivo distribution and clearance properties.
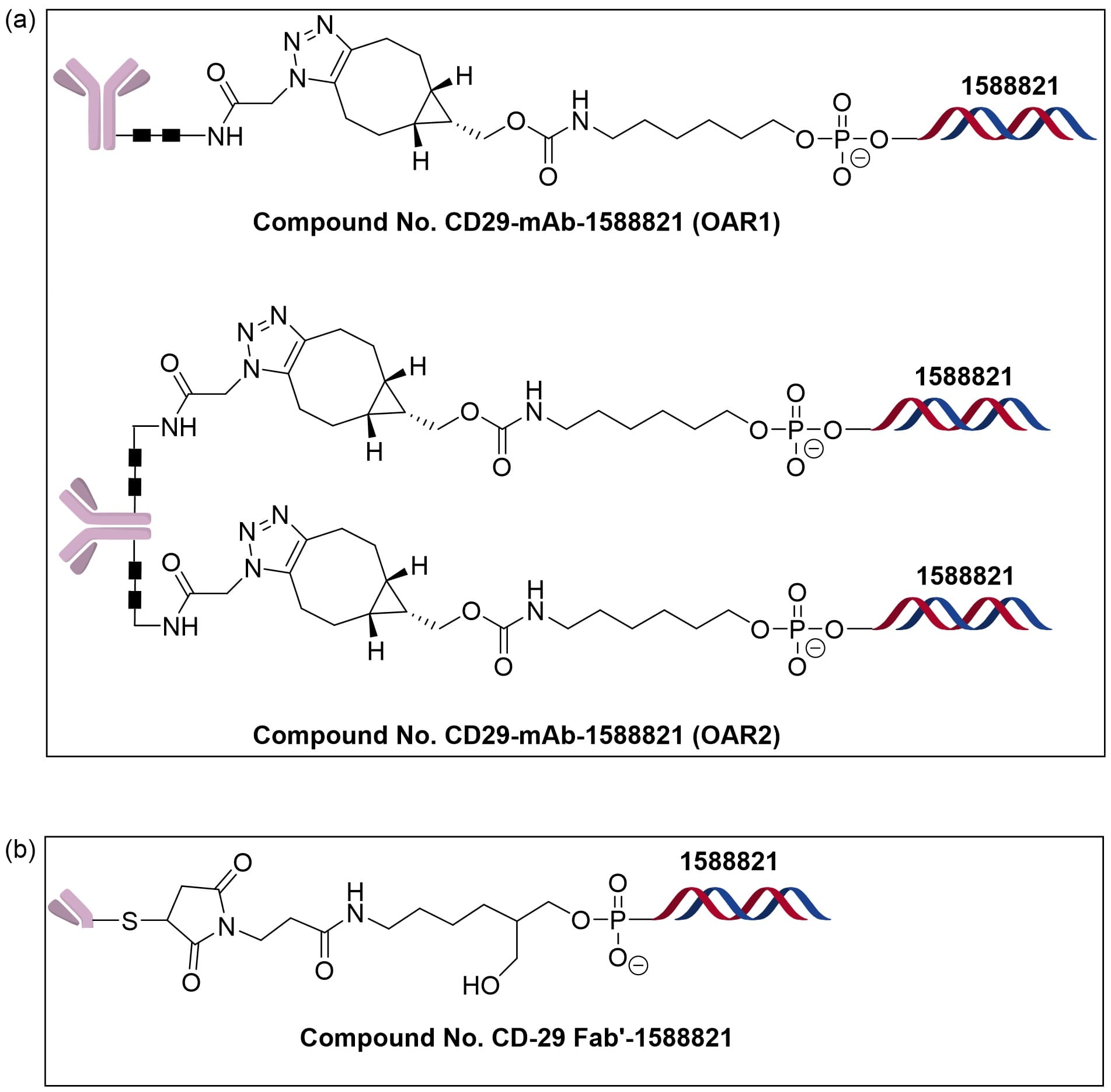
Figure 3. Antibody–oligonucleotide conjugates(AOCs) prepared from anti-CD29 in different formats: full-length antibody (a) and Fab (b)
Taken together, antibodies provide the core targeting and delivery functions in AOCs, but differences in target choice, antibody scaffold/format and engineering, and conjugation processes can substantially affect an AOC’s pharmacokinetics (half-life, tissue distribution, internalization pathways, etc.) and immunological safety. Successful AOC design requires systematic trade-offs among targeting, payload release within endosomes/lysosomes and lysosomal-escape efficiency after receptor-mediated internalization, and the overall ADME and safety profile. Common complementary optimization strategies include receptor selection and affinity tuning, antibody scaffold engineering (e.g., retaining or removing Fc functions, glycosylation adjustment), site-specific conjugation, and chemical linker design to achieve the best balance of efficacy and safety.
Oligonucleotide Payload in Antibody–Oligonucleotide Conjugates (AOCs)
As shown in Table 1, the oligonucleotides commonly used in investigational AOCs are siRNAs and ASOs. siRNAs are roughly 20–25 nucleotides long, double-stranded RNA, whereas ASOs are single-stranded and typically about 15–25 nucleotides in length. Both are built from bases, sugars (ribose or deoxyribose), and a phosphate backbone; unmodified ASOs and siRNAs are poorly stable in the biological matrices. However, their mechanisms of action differ substantially. Below, we describe the common chemical modifications used to improve stability and discuss how to choose between siRNA and ASO in AOC design.
Chemical modifications of oligonucleotides
Oligonucleotides’ intrinsic negative charge, high polarity, relatively large molecular weight, and susceptibility to nucleases render them vulnerable to enzymatic degradation, poor membrane permeability, and immune recognition in vivo, limiting their stability and targeting efficiency. Chemical modifications can substantially enhance their stability [10]. Although conjugation to an antibody offers some protection, Toshima et al. [6] reported that the siRNA segments in their AOC constructs underwent hydrolysis in plasma and tumor tissue, which could undermine siRNA endosomal escape and target enrichment. This indicates that oligonucleotides in AOC still require chemical modification to improve stability. The main modification sites are the phosphate backbone, the sugar ring, and the bases (Figure 4); the common modifications and their effects are summarized in Table 2 [10]. Rational combinations of these modification strategies are an important approach to optimizing AOC efficacy.
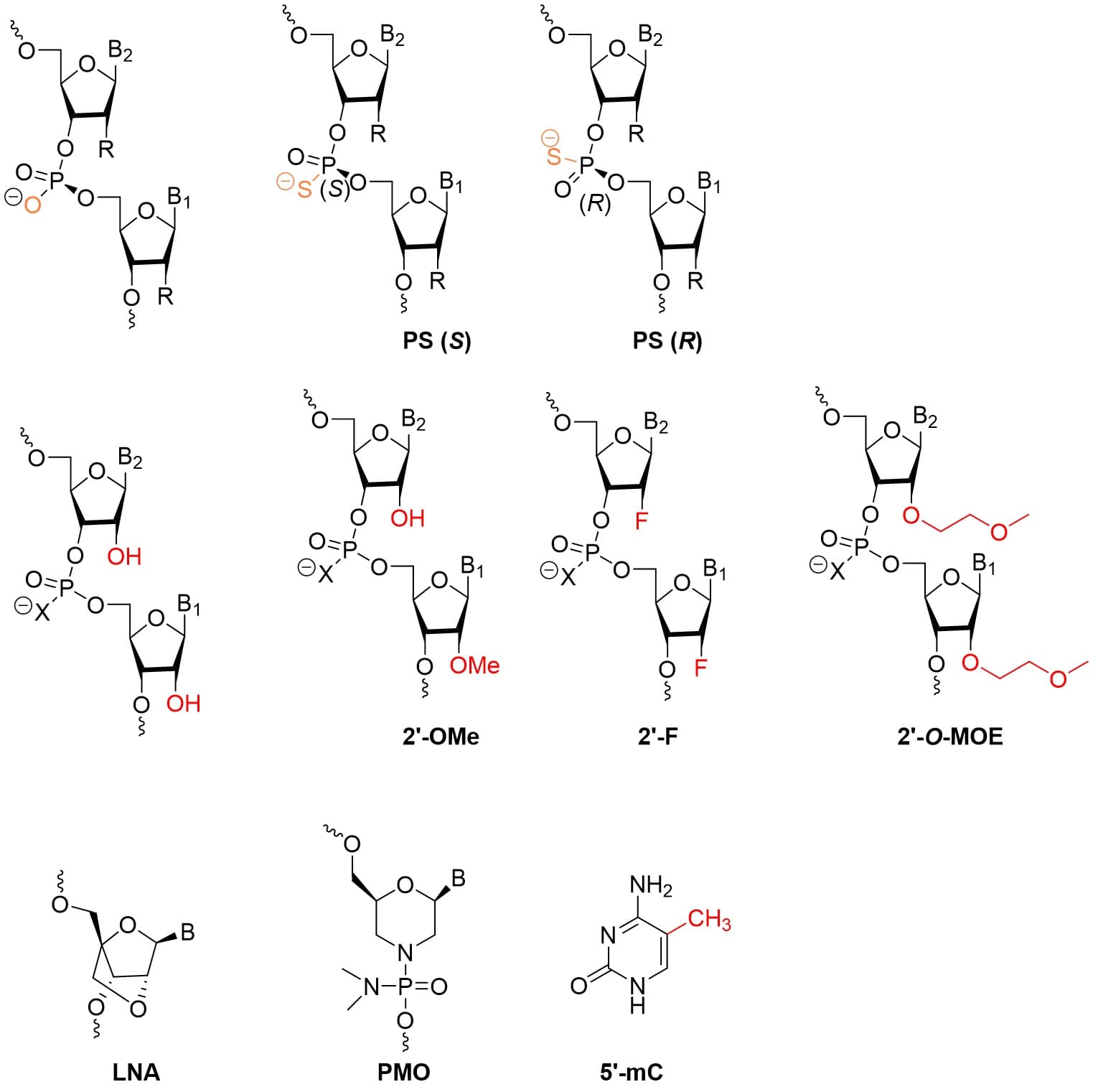
Figure 4. Common chemical modification strategies for oligonucleotides.
Table 2. Main types of oligonucleotide modifications and their functions
Modification Site | Common Modifications | Function |
Sugar Ring (2'-position) | 2'-O-Methyl (2'-OMe) | Enhances nuclease resistance, reduces immune stimulation |
2'-Fluoro (2'-F) | Increases thermal stability (Tm value) | |
Locked Nucleic Acid (LNA) | Increases hybridization affinity | |
Phosphate Backbone | Phosphorothioate (PS) | Resists nuclease degradation, enhances protein binding |
Phosphorodiamidate Morpholino Oligomer (PMO) | Neutral charge reduces non-specific interactions | |
Base | 5-Methylcytosine (5-mC) | Enhances C–G pairing stability and can reduce immune recognition. |
Selection of oligonucleotide
In current AOC drug development, different oligonucleotide modalities can be used against the same target. For example, Avidity’s AOC 1001 uses siRNA, whereas Dyne’s Dyne-101 employs an antisense oligonucleotide (ASO). Both target mutant DMPK to disrupt the hairpin structures formed by CUG repeats and to restore the function of muscleblind-like protein (MBNL), thereby treating myotonic dystrophy type 1 (DM1). The main differences are:
AOC 1001 (siRNA): siRNAs are negatively charged, hydrophilic, double-stranded RNAs that poorly cross cell membranes and have limited nuclear localization. They typically act in the cytoplasm, where they are loaded into Ago2-containing RISC complexes to mediate target mRNA cleavage. Avidity’s public materials emphasize DMPK knockdown but do not specify a defined subcellular site of action.
Dyne-101 (ASO): ASOs are single-stranded oligonucleotides that can access the nucleus and tend to be more hydrophobic. Dyne’s strategy is based on the retention of mutant DMPK mRNA in the nucleus; ASOs can enter the nucleus, bind the target mRNA directly, and recruit RNase H to induce degradation.
The two companies’ choices of oligonucleotide modality reflect different AOC design strategies, but ultimate efficacy must be confirmed by clinical and translational studies. When deciding between siRNA and ASO, multiple factors should be weighed, including physicochemical properties, cellular and subcellular distribution, potency, endosomal escape, and safety. Figure 5 provides a visual comparison of the typical intracellular distribution and mechanisms of action of the two oligonucleotide classes.
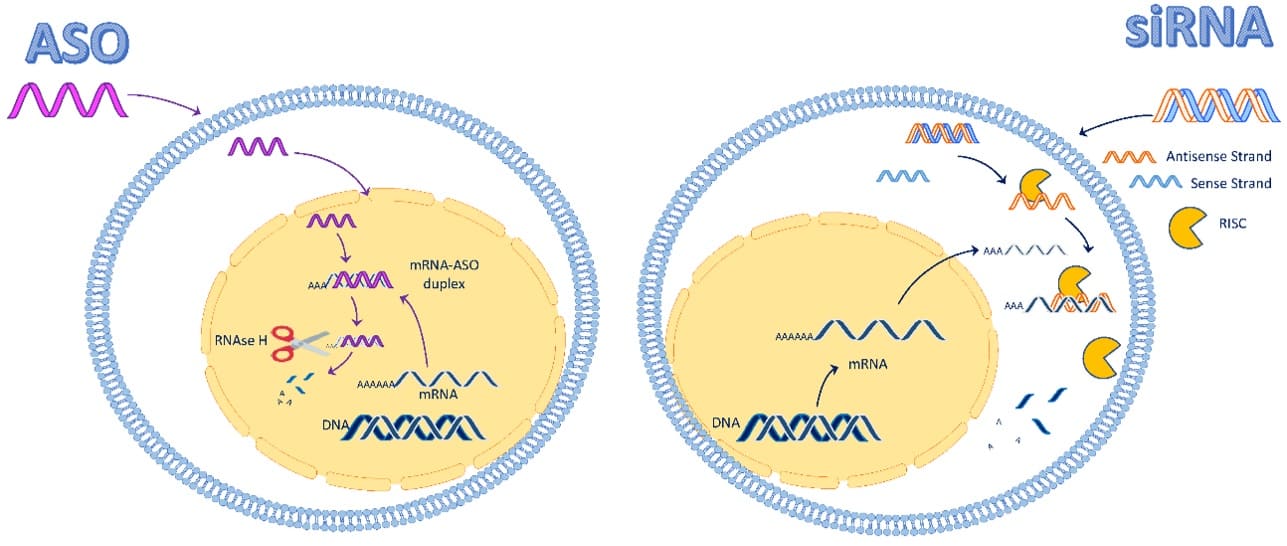
Figure 5. Intracellular distribution and mechanisms of action of siRNA and ASO [11]
Concluding Remarks
This review summarizes recent progress in the development of investigational antibody–oligonucleotide conjugates (AOCs), with a particular focus on the structure–function relationships of the antibody and oligonucleotide components. Antibody structure, the conjugation site and strategy (including the linker chemistry and OAR) together influence circulation half-life, tissue distribution, and receptor–mediated endocytosis. Oligonucleotides typically have relatively limited in vivo stability; their fate is governed largely by backbone chemistry and sugar/base modifications, strand type and length, interactions with proteins, and the conjugation strategy to the antibody carrier. Choice of siRNA versus ASO should therefore be balanced against each modality’s intracellular distribution and the pathogenic mechanism of the target gene.
A deep understanding of the structural features of both the antibody and the oligonucleotide, and how these features affect ADME, is fundamental to the design and clinical translation of AOC. Although AOCs that combine the strengths of both molecular classes offer new opportunities for cross-tissue delivery, their structural complexity complicates metabolic pathways and analytical workflows, so thorough structural characterization is essential to support ADME investigations and to inform the design and interpretation of PK studies. Notably, the linker, the chemical bridge between antibody and oligonucleotide, has a profound impact: its chemistry, conjugation site, and release mechanism strongly influence AOC stability, target-site release efficiency, and in vivo metabolic fate.
Leveraging its understanding of AOC structural features and ADME/PK properties, WuXi AppTec DMPK has established an integrated DMPK research platform. This platform enables developers at different stages to more efficiently and accurately characterize both in vitro and in vivo ADME profiles of AOC candidates, to better elucidate the relationships between efficacy and safety, and to provide robust support for subsequent safety assessments and clinical translation.
Authors: Yong Qin, Liqi Shi, Hong Zhang, Weiqun Cao
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Z. Xiaoxia, L. Dongke, G. Linjie, L. Ji, C. Hongxu, and G. Lihai, “Application of Capillary Zone Electrophoresis (CZE) and High Resolution Mass Spectrometry (HRMS) in the components separation and structures confirmation of Antibody- oligonucleotide conjugates (AOC),” pp. 1–4, 2021.
[2] M. A. Jian, Y. U. Tonglin, C. U. I. Shuaishuai, H. E. Yujian, and W. U. Li, “Research Advances in Oligonucleotide Conjugate,” vol. 48, no. 8, pp. 565–578, 2024, doi: 10.20053/j.issn1001-5094.2024.08.002.
[3] S. J. Barker et al., “Targeting the transferrin receptor to transport antisense oligonucleotides across the mammalian blood-brain barrier,” Sci. Transl. Med., vol. 16, no. 760, 2024, doi: 10.1126/scitranslmed.adi2245.
[4] M. Kothari et al., “A Comprehensive Review of Monoclonal Antibodies in Modern Medicine: Tracing the Evolution of a Revolutionary Therapeutic Approach,” Cureus, vol. 16, no. 6, 2024, doi: 10.7759/cureus.61983.
[5] T. T. Hansel, H. Kropshofer, T. Singer, J. A. Mitchell, and A. J. T. George, “The safety and side effects of monoclonal antibodies,” Nat. Rev. Drug Discov., vol. 9, no. 4, pp. 325–338, 2010, doi: 10.1038/nrd3003.
[6] A. Toshima, Y. Shiraishi, D. Shinmi, Y. Kagawa, and J. Enokizono, “Comprehensive Analyses of the Intracellular and in Vivo Disposition of Fab– Small Interfering RNA Conjugate to Identify Key Issues to Improve Its in Vivo Activity,” Drug Metab. Dispos., vol. 51, no. 3, pp. 338–347, 2023, doi: 10.1124/dmd.122.001098.
[7] E. Holz, M. Darwish, D. B. Tesar, and W. Shatz-Binder, “A Review of Protein- and Peptide-Based Chemical Conjugates: Past, Present, and Future,” Pharmaceutics, vol. 15, no. 2, 2023, doi: 10.3390/pharmaceutics15020600.
[8] H. Lulu, R. Alexey, P. T. P, and N. Mehran, “CD29 Targeted Oligonucleotides and Uses Thereof,” WO2025/006955A2, 2025
[9] M. Cochran et al., “Structure-Activity Relationship of Antibody-Oligonucleotide Conjugates: Evaluating Bioconjugation Strategies for Antibody-siRNA Conjugates for Drug Development,” J. Med. Chem., vol. 67, no. 17, pp. 14852–14867, 2024, doi: 10.1021/acs.jmedchem.4c00802.
[10] M. Egli and M. Manoharan, “Chemistry, structure and function of approved oligonucleotide therapeutics,” Nucleic Acids Res., vol. 51, no. 6, pp. 2529–2573, 2023, doi: 10.1093/nar/gkad067.
[11] C. Gareri, A. Polimeni, S. Giordano, L. Tamm, A. Curcio, and C. Indolfi, “Antisense Oligonucleotides and Small Interfering RNA for the Treatment of Dyslipidemias,” J. Clin. Med., vol. 11, no. 13, p. 3884, 2022.
Related Services and Platforms




-

 MetID (Metabolite Profiling and Identification)Learn More
MetID (Metabolite Profiling and Identification)Learn More -

 Novel Drug Modalities DMPK Enabling PlatformsLearn More
Novel Drug Modalities DMPK Enabling PlatformsLearn More -

 In Vitro MetID (Metabolite Profiling and Identification)Learn More
In Vitro MetID (Metabolite Profiling and Identification)Learn More -

 In Vivo MetID (Metabolite Profiling and Identification)Learn More
In Vivo MetID (Metabolite Profiling and Identification)Learn More -

 Metabolite Biosynthesis and Structural CharacterizationLearn More
Metabolite Biosynthesis and Structural CharacterizationLearn More -

 Metabolites in Safety Testing (MIST)Learn More
Metabolites in Safety Testing (MIST)Learn More -

 PROTAC DMPK ServicesLearn More
PROTAC DMPK ServicesLearn More -

 ADC DMPK ServicesLearn More
ADC DMPK ServicesLearn More -

 Oligo DMPK ServicesLearn More
Oligo DMPK ServicesLearn More -

 PDC DMPK ServicesLearn More
PDC DMPK ServicesLearn More -

 Peptide DMPK ServicesLearn More
Peptide DMPK ServicesLearn More -

 mRNA DMPK ServicesLearn More
mRNA DMPK ServicesLearn More -

 Covalent Drugs DMPK ServicesLearn More
Covalent Drugs DMPK ServicesLearn More
Stay Connected
Keep up with the latest news and insights.