Conjugated drugs, due to their clinical outcomes and potential commercial value, have attracted wide industry attention and have become a hot field in pharmaceutical research and development. Among them, the most mature class is antibody–drug conjugates (ADCs). From the FDA's approval of Mylotarg® for adult acute myeloid leukemia in 2000 to the approval of Sacituzumab Tirumotecan by the NMPA in November 2024, 16 ADC drugs have been approved globally, covering multiple targets in solid tumors and hematological malignancies. Other conjugate modalities are also rapidly emerging, including peptide–drug conjugates (PDCs), antibody–peptide conjugates (APCs), antibody–oligonucleotide conjugates (AOCs), antibody‑conjugated degraders (DACs), radioligand conjugates (RDCs), and small-molecule drug conjugates (SMDCs). With rapid iteration of conjugation technologies, the field has entered a flourishing era of diverse conjugated therapeutics. This article introduces APC drugs and their pharmacokinetic research, aiming to deepen the understanding of APC drugs and provide references for APC pharmacokinetic studies.
What Are Antibody–Peptide Conjugates (APCs)
APC typically consists of an antibody, a peptide, and a linker. In February 2024, Amgen published Phase I clinical results in Nature Metabolism for its investigational APC drug AMG 133 (a GIPR mAb-GLP-1 analog conjugate) for the obesity indication [1]. The results showed that obese patients required only one injection per month, and following 85 days of high-dose AMG 133 treatment, their body weight decreased by 14.5% (approximately 13 kg). Remarkably, 150 days after stopping treatment, patients still maintained an 11.2% weight loss. These results demonstrate the strong potential of AMG 133 in the field of obesity treatment and indicate that achieving breakthrough advancements are being made in the APC drug field.
Based on their composition, APCs can be classified into: 1) APCs with the peptide as the effector component; 2) APCs with the antibody as the effector component; and 3) APCs where both the antibody and peptide are effector components. For the first type of APC (as shown in Figure 1A), the antibody part primarily functions in targeting, improving the stability of the peptide drug, prolonging half-life, increasing peptide accumulation at the target site, and reducing systemic toxicity. The second type of APC (as shown in Figure 1B) consists of a homing peptide or other functional peptide, a linker, and a therapeutic antibody, mainly used to improve poor tissue penetration and uneven distribution of antibodies, thereby enhancing the therapeutic efficacy and safety of antibody administration. The mechanism of the third type of APC (as shown in Figure 1C) is similar to that of bi-/multi-specific antibodies, where both the antibody and peptide are specific molecules that can act on different targets of the same disease to exert blocking or agonistic effects, achieving synergistic therapeutic outcomes. Currently, information on some reported APC drugs is summarized in Table 1.
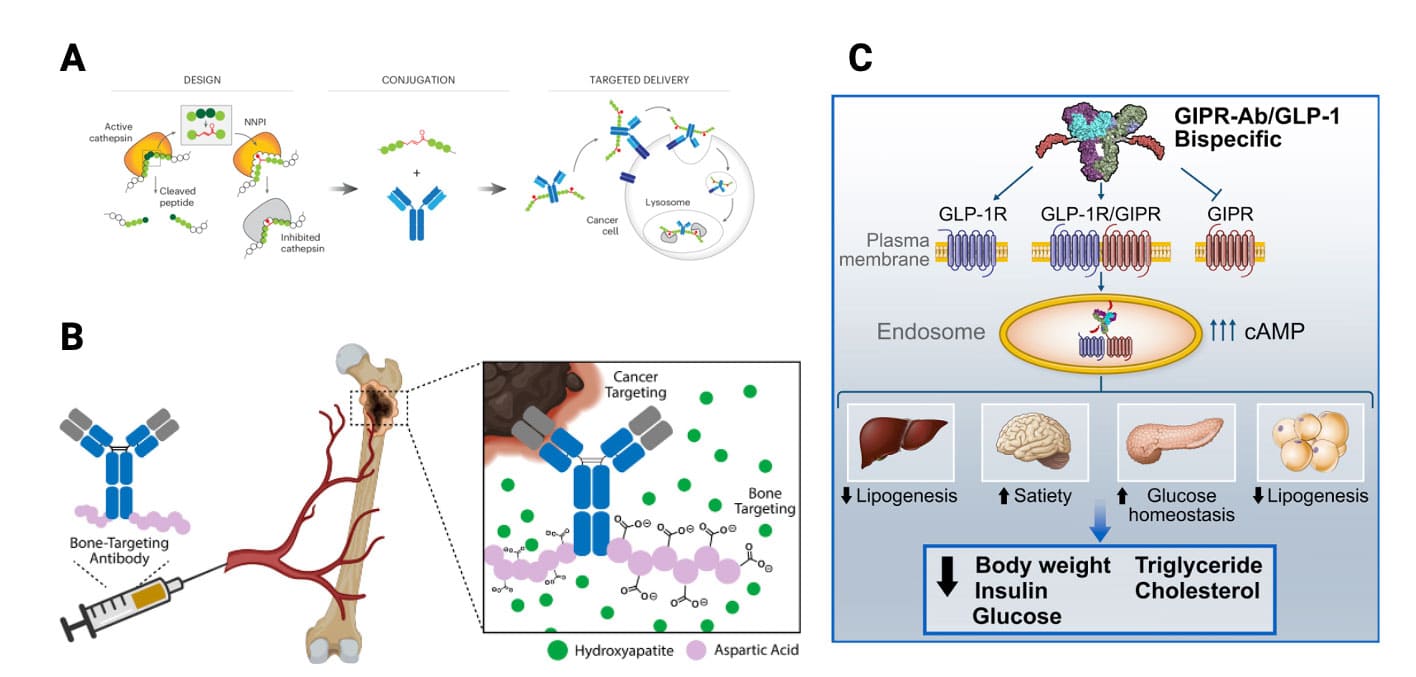
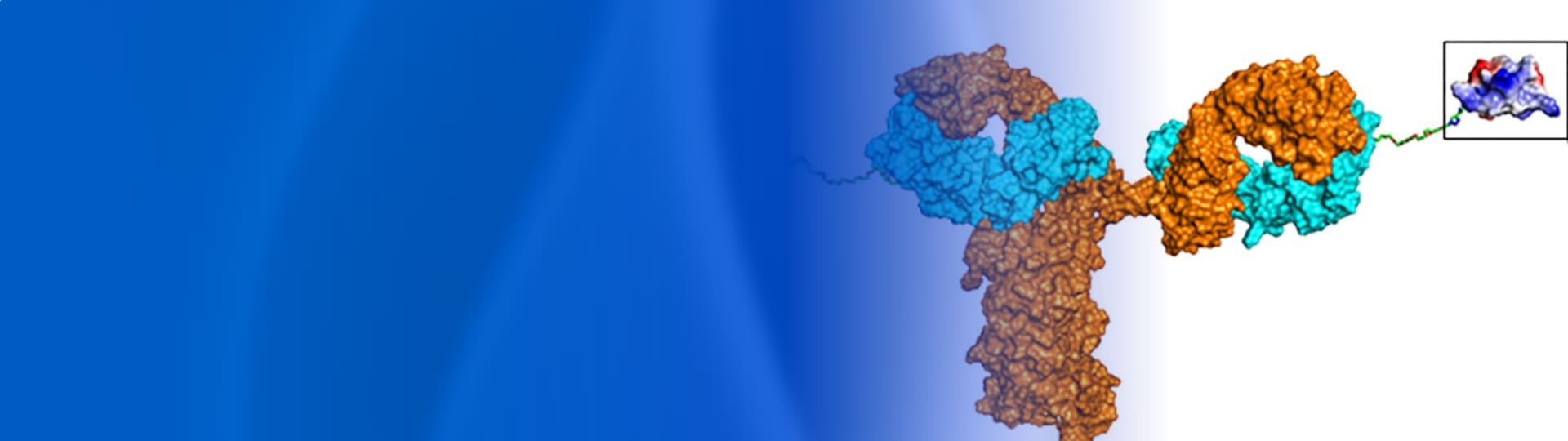
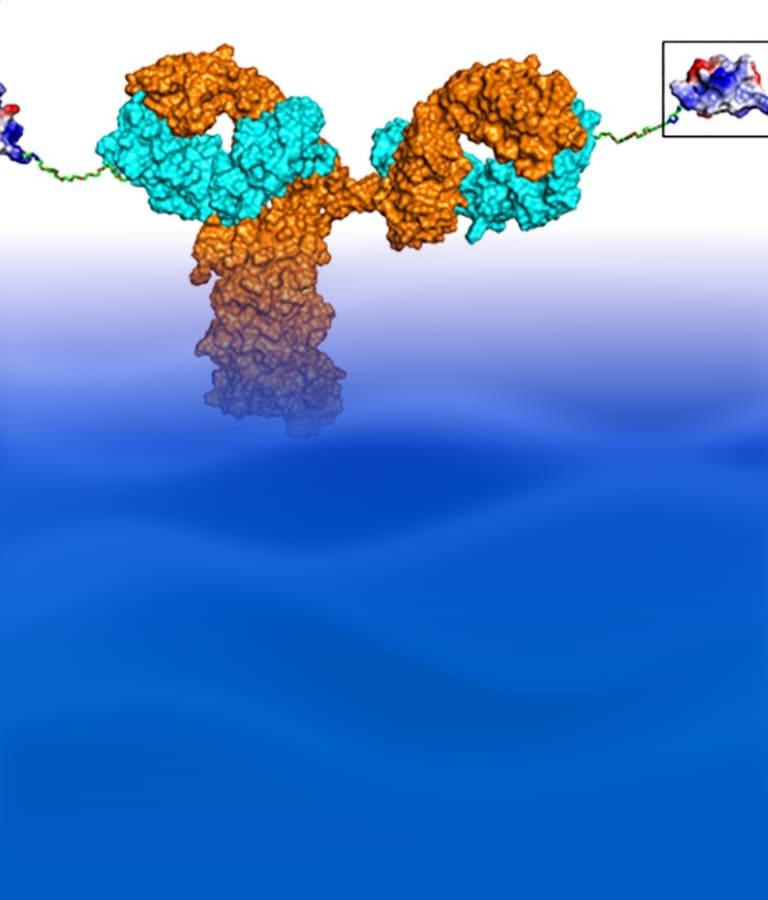
Figure 1. Different types of antibody–peptide conjugates (APC) drugs. (A) Tumor-targeting antibody-cathepsin peptide inhibitor conjugate [2]; (B) Bone-homing peptide-trastuzumab conjugate [3]; (C) GIPR-Ab/GLP-1 analog conjugate [4]
Table 1. Summary of information on seven reported APC drugs
APC Name | Developing Company | Development Stage | Antibody | Peptide | Effector Component | Therapeutic Area | Administration | References |
AMG 133 | Amgen | Phase 2 | GIPR-Ab | GLP-1 analog | Peptide × Antibody | Obesity | IV, SC | [1] |
AMG 570 | Amgen | Phase 2 | ICOSL-Ab | BAFF inhibitory peptide | Peptide × Antibody | Systemic Lupus Erythematosus, Rheumatoid Arthritis | IV, SC | [5,6] |
CX-072 | CytomX | Phase 2 | PD-L1-Ab | Masking peptide | Antibody | Oncology | IV | [7,8] |
Antibody-JzTx-V peptide conjugate | Amgen | Preclinical | αDNP-Ab | JzTx-V series peptide | Peptide | Pain | IV, SC | [9] |
CI107 | CytomX | Preclinical | Ab (EGFR×CD3) | Masking peptide | Antibody | Oncology | IV | [10] |
EGFR mAb prodrug | CytomX | Preclinical | EGFR-Ab (Cetuximab) | Masking peptide | Antibody | Oncology | IV, IP | [11] |
Antibody-oxyntomodulin analog conjugate | Janssen | Preclinical | Immunologically silent IgG4 IgG4 | Oxyntomodulin analog | Peptide | Obesity | SC | [12] |
Note: Ab: Antibody; GIPR: Glucose-dependent Insulinotropic Polypeptide Receptor; GLP-1: Glucagon-like peptide-1; ICOSL: Inducible T-cell Costimulator Ligand; PD-L1: Programmed Death-Ligand 1; DNP: 2,4-Dinitrophenol; EGFR: Epidermal Growth Factor Receptor; CD3: Cluster of Differentiation 3; IV: Intravenous injection; SC: Subcutaneous injection; IP: Intraperitoneal injection.
Pharmacokinetic Properties of APC Drugs
The pharmacokinetic process of APC drugs is influenced by their three structural components. The antibody serves as the main part, inherently possessing PK characteristics such as low clearance, long half-life, and low apparent volume of distribution. However, the overall pharmacokinetic profile of APC drugs is also affected by the linker, the peptide, and the drug-to-antibody ratio (DAR).
Absorption
Due to their large molecular weight, APC drugs struggle to cross the gastrointestinal barrier. They are also susceptible to hydrolysis and protein denaturation in the gastrointestinal environment, making oral administration unfeasible. As shown in the table above, the currently developed administration routes for APC drugs are primarily intravenous and subcutaneous injection. Intravenous injection does not involve an absorption process. Subcutaneously injected antibody drugs are generally first absorbed via the lymphatic system before being transported into the systemic circulation.
Distribution
The in vivo distribution of APC drugs is mainly influenced by the distribution of target receptors and blood flow to tissues and organs. After entering the systemic circulation, APC drugs need to cross the vascular wall to reach the target tissue and exert their effect. Similar to large molecules like antibodies, APC drugs may enter the interstitium through convection in blood-tissue fluid or via transcytosis across vascular endothelial cells, and then proceed from the extracellular matrix to the target site. Most APC drugs contain targeting functional components, such as antibodies or homing peptides. APC drugs can directly bind to cell surface antigens or receptors, or they can dissociate extracellularly at the target tissue under specific pH conditions or the action of hydrolases. Overall, due to their large molecular size and poor lipophilicity, APC drugs generally have a low apparent volume of distribution.
Metabolism
Antibody–peptide conjugates undergo three main metabolic pathways in the body.
Target-mediated specific drug disposition (TMDD)
Target-mediated specific metabolism refers to the process where the Fab region of the antibody, part of the APC, or the specific amino acid sequence of the peptide binds to antigens or receptors on the target cell surface, is internalized into the cell, and is degraded into peptide fragments or amino acids in the lysosome. Because the number of sites for cell surface antigens/receptors is limited, this pathway is saturable and causes the PK of APCs to exhibit nonlinear characteristics, meaning clearance decreases with increasing dose, and half-life increases with increasing dose. When the dose is sufficiently high, this elimination pathway becomes non-dominant, and nonlinear PK transitions to linear PK. Additionally, a small fraction of cell surface antigens may be shed and become soluble antigens; the antibody part of the APC can also bind to these antigens, forming immune complexes that are eventually eliminated.
Non-specific metabolism pathways
APCs can enter cells via non-specific pinocytosis or Fcγ receptor-mediated endocytosis by cells such as macrophages and natural killer cells. A portion of the internalized APC molecules is degraded into peptide fragments or amino acids under the action of lysosomes; another portion of APCs, under weakly acidic conditions, binds to the neonatal Fc receptor (FcRn) via the Fc region and is transported back to the cell surface, escaping degradation, thereby reducing in vivo clearance and prolonging biological half-life. Non-specific metabolic pathways may lead to the release of the attached peptide drug at non-intended target sites, causing off-target side effects. Other non-specific metabolic pathways include the cleavage of masking peptides and some linkers under specific pH environments or by the action of certain hydrolases (e.g., the slightly acidic microenvironment of tumor tissues and matrix metalloproteinases).
Immunogenicity of APC drugs
Similar to other macromolecular drugs, APCs may cause immunogenicity, stimulating the body to produce anti-drug antibodies (ADA). ADA can specifically bind to the drug, forming complexes that are subsequently eliminated by the immune system; this neutralizing effect becomes another clearance mechanism for APCs.
Excretion
Intact APC drugs, due to their large molecular weight, cannot be directly filtered and cleared by the glomerulus. The peptide drug, peptide fragments, and amino acids produced after APC metabolism may fall below the glomerular filtration threshold (60 kDa) and be excreted via the kidneys. Additionally, these low molecular weight metabolites may also be excreted through bile via transporter-mediated processes.
Case Studies in APC Pharmacokinetic Research
AMG 133 (Maridebart cafraglutide)
AMG 133 is a GIPR/GLP-1R bispecific molecule developed by Amgen (Figure 2). Preclinical and clinical studies have shown that AMG 133 has acceptable safety and tolerability and exhibits significant dose-dependent weight loss effects. In the clinical multiple ascending dose cohorts, weight loss was maintained for 150 days after the last administration [1].
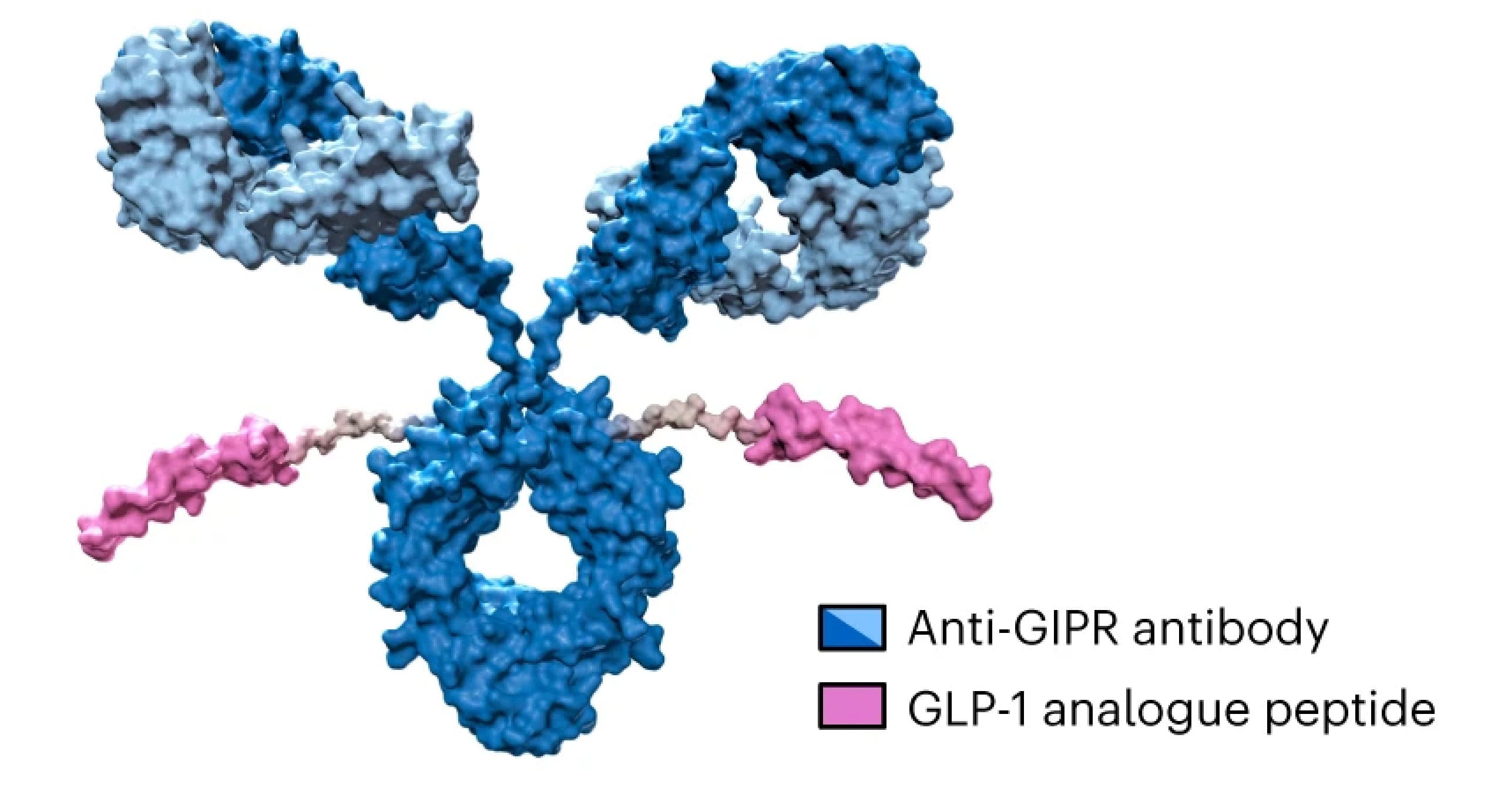
Figure 2. Structure of AMG 133 [1]
Preclinical pharmacokinetics of AMG 133
Preclinical PK studies of AMG 133 were conducted in CD-1 mice and normal and obese cynomolgus monkeys. Concentrations of intact AMG 133 (GIPR-Ab with at least one GLP-1 analog peptide attached) and total antibody (GIPR-Ab with or without GLP-1 analog peptides) in plasma were measured using ELISA methods, both with an assay range of 30–2000 ng/mL.
After a single IV dose of 5 mg/kg in mice, the C0 values for intact AMG 133 and total antibody were similar (127 vs 109 μg/mL), but intact AMG 133 had faster clearance (CL) (0.568 vs 0.367 mL/h/kg), a shorter terminal half-life (t1/2) (118 vs 206 h), and lower exposure (AUC0-inf) (8840 vs 13700 μg·h/mL), as shown in Table 2.
PK parameters after a single SC dose of 3 mg/kg AMG 133 in cynomolgus monkeys are shown in Table 3. Similar to the PK data in CD-1 mice, intact AMG 133 had a shorter t1/2 (207 vs 292 h) and lower exposure (AUC0-inf) (9110 vs 15300 μg·h/mL) compared to total antibody.
PK parameters after multiple SC administrations (once weekly for 6 weeks) of AMG 133 in obese male cynomolgus monkeys are shown in Table 4. Within the dose range of 0.5 mg/kg to 1 mg/kg, AMG 133 exhibited a long half-life, with intact AMG 133 and total antibody ranging from 189–222 h and 231–284 h, respectively. Plasma exposure for both intact AMG 133 and total antibody increased with increasing dose. Compared to PK data from non-obese monkeys, dose-normalized plasma exposure was lower in obese cynomolgus monkeys.
Table 2. PK parameters of AMG 133 after a single IV dose of 5 mg/kg in CD-1 mice [1]
AMG 133 | C0 (μg/mL) | t1/2 (h) | AUC0-inf (μg∙h/mL) | Vss (mL/kg) | CL (mL/h/kg) |
Total | 109 | 206 | 13700 | 103 | 0.367 |
Intact | 127 | 118 | 8840 | 83.4 | 0.568 |
Table 3. PK parameters of AMG 133 after a single SC dose of 3 mg/kg in female cynomolgus monkeys [1]
AMG 133 | Cmax (μg/mL) | t1/2 (h) | tmax (h) | AUC0-last (μg∙h/mL) | AUC0-inf (μg∙h/mL) | CL/F (mL/h/kg) |
Total | 30.4 | 292 | 48 | 13200 | 15300 | 0.198 |
Intact | 24.8 | 207 | 96 | 8550 | 9110 | 0.334 |
Table 4. PK parameters of AMG 133 after consecutive SC doses in male obese cynomolgus monkeys [1]
AMG 133 | Dose (mg/kg) | Cmax (μg/mL) | t1/2 (h) | tmax (h) | AUC0-last (μg∙h/mL) | AUC0-inf (μg∙h/mL) | CL/F (mL/h/kg) |
Total | 1 | 4.79 | 231 | 96 | 1880 | 2270 | 0.456 |
0.5 | 3.59 | 284 | 168 | 1450 | 1900 | 0.277 | |
0.75 | 4.18 | 258 | 168 | 1620 | 2020 | 0.380 | |
Intact | 1 | 4.34 | 189 | 96 | 1400 | 1580 | 0.673 |
0.5 | 2.87 | 222 | 96 | 977 | 1160 | 0.461 | |
0.75 | 3.13 | 191 | 96 | 1030 | 1160 | 0.656 |
Clinical pharmacokinetics of AMG 133
Participants with obesity were randomly assigned in a 3:1 ratio to receive AMG 133 or a placebo. The single ascending dose (SAD) trial evaluated AMG 133 in 6 cohorts over a dose range of 21–840 mg. The multiple ascending dose (MAD) trial evaluated doses in 3 cohorts ranging from 140–420 mg, administered every 4 weeks on days 1, 29, and 57. AMG 133 or placebo was administered subcutaneously. During the clinical phase, intact AMG 133 and total antibody were measured using an LC-MS/MS method with a lower limit of quantitation of 50 ng/mL.
In all SAD cohorts, AMG 133 reached maximum concentration approximately 4–7 days (median tmax) after administration. The mean t1/2 for intact AMG 133 and total antibody were approximately 14–16 days and 21–24 days, respectively. In the MAD cohorts, the median tmax range was 4–6 days post-dose, consistent with the SAD cohorts. After the last dose on day 57, the exposure (AUC0-28) for intact AMG 133 and total antibody is shown in Table 6.
Due to the synergistic effects of GIPR antagonism and GLP-1R agonism, AMG 133 demonstrated superior weight loss efficacy compared to single-target agents in both preclinical and clinical experiments. Furthermore, the conjugation of the peptide to the antibody significantly extended the half-life of the GLP-1-based drug, supporting a once-monthly dosing frequency. This offers improved patient compliance and convenience compared to current long-acting GLP-1 formulations like Wegovy and Zepbound (administered once weekly).
Table 5. PK parameters of AMG 133 from the clinical SAD cohorts [1]
| tmax (days) | t1/2 (days) | |||
Dose (mg) | N | Intact AMG 133 | Total AMG 133 | Intact AMG 133 | Total AMG 133 |
21 | 6 | 5 | 5.9 | 14.3 | 21 |
840 | 6 | 5.4 | 5.5 | 16.5 | 23.8 |
Table 6. PK parameters of AMG 133 from the clinical MAD cohorts [1]
Dose (mg) | N | Intact AMG 133 AUC0-28 (day∙μg/mL) | Total AMG 133 AUC0-28 (day∙μg/mL) |
140 | 6 | 214 | 297 |
280 | 5 | 443 | 599 |
420 | 3 | 1220 | 1610 |
AMG 570 (Rozibafusp alfa)
AMG 570 is another APC drug developed by Amgen, composed of the inducible T-cell costimulator ligand (ICOSL) antagonist antibody AMG 557 conjugated to a B-cell activating factor (BAFF) inhibitory peptide. It simultaneously inhibits both 1) the ICOSL signaling pathway that stimulates T cells and 2) the BAFF signaling pathway that stimulates B cells, showing broad prospects for treating autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis. It is currently in Phase II clinical development.
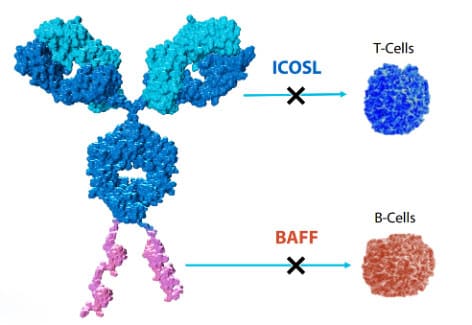
Figure 3. Structure of AMG 570 [13]
Preclinical pharmacokinetics of AMG 570
PK studies of AMG 570 were conducted in cynomolgus monkeys[6]. Male monkeys (n=4) received a single IV dose of 10 mg/kg. Total antibody in serum was detected using anti-human IgG capture and detection, while intact AMG 570 concentration was detected using ICOSL-Fc capture and biotinylated BAFF. The results showed that the level and trend of intact AMG 570 in serum were similar to those of the total antibody, see Figure 4. Furthermore, the PK characteristics of AMG 570 and the ICOSL antibody AMG 557 were compared in cynomolgus monkeys, revealing that the PK parameters of AMG 570 and AMG 557 were relatively close, see Table 7.
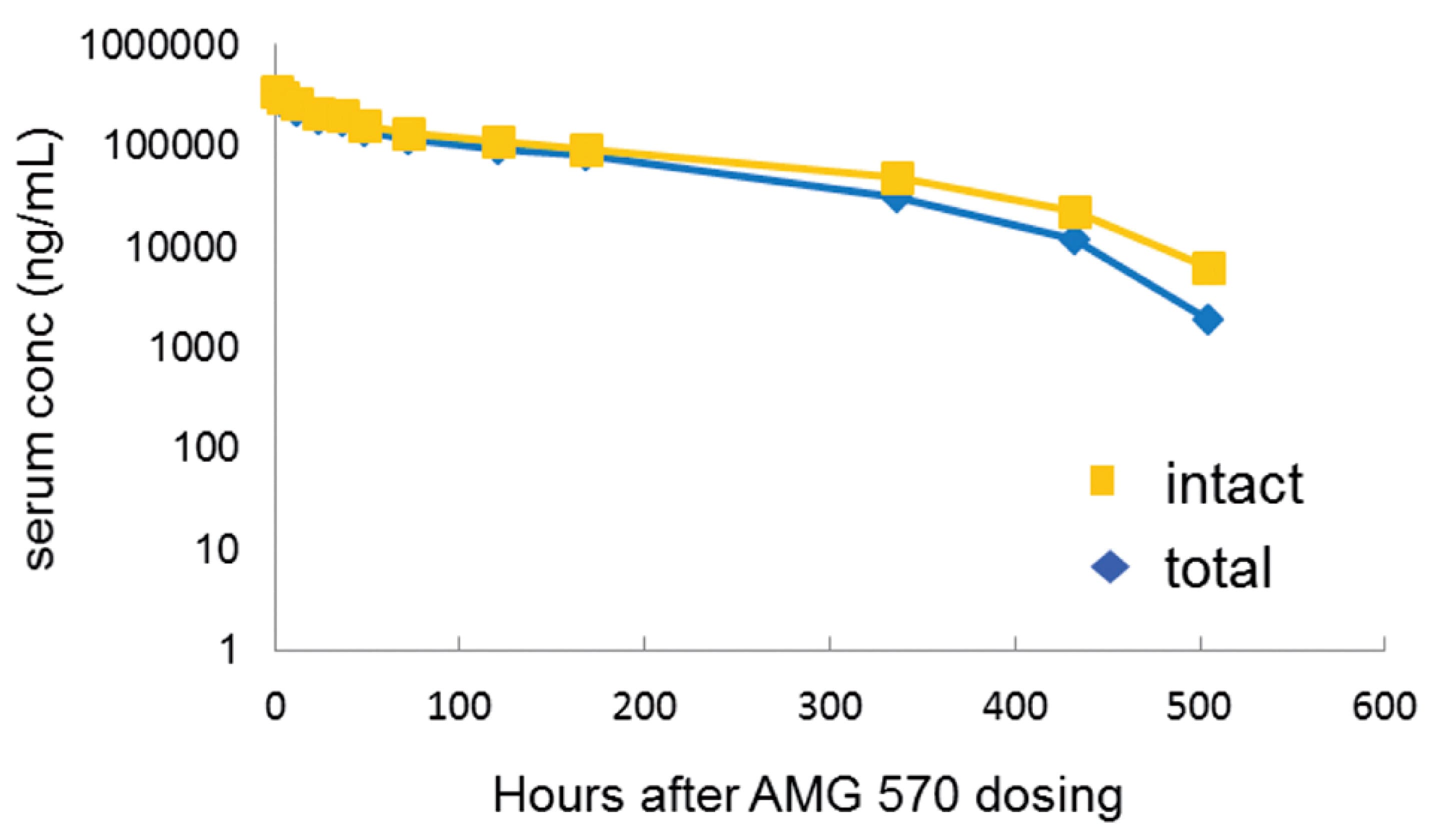
Figure 4. PK profile of AMG 570 after a single IV dose of 10 mg/kg in cynomolgus monkeys (n=4) [6]
Table 7. Comparison of PK parameters between AMG 570 and AMG 557 in cynomolgus monkeys [6]
Parameter | AMG 570 | AMG 557 | ||
Dosing Regimen | 10mpk IV | 10mpk SC | 10mpk IV | 10mpk SC |
Cmax (μg/mL) | 323 | 90 | 264 | 112 |
Tmax (h) | -- | 45 | -- | 72 |
AUC0-inf (μg∙h/mL) | 33800 | 20300 | 26100 | 23800 |
MRT0-inf (h) | 136 | 132 | 138 | 144 |
CL (mL/h/kg) | 0.303 | 0.491 | 0.388 | 0.427 |
Vss (mL/kg) | 42.5 | -- | 52.1 | -- |
Clinical pharmacokinetics of AMG 570
Participants were randomly assigned in a 3:1 ratio to receive AMG 570 or placebo [7]. The SAD study evaluated AMG 570 in 7 cohorts over a dose range of 7–700 mg. The MAD study evaluated doses in 4 cohorts ranging from 70–420 mg, administered once every two weeks for a total of 6 doses. AMG 570 or placebo was administered subcutaneously. AMG 570 was measured using an ELISA method with a quantitation range of 10-5,000 ng/mL.
In the SAD study, PK exhibited nonlinearity. The median tmax was 3 to 5 days post-dose. The mean t1/2 ranged from 3.21 days (7 mg) to 7.00 days (700 mg), with a trend toward longer t1/2 at higher doses. Clearance (CL/F) also decreased with increasing dose. Within the 7-700 mg dose range, Cmax and AUC0-inf increased greater than dose-proportionally, see Table 8. In the MAD study, after 6 q2w doses, the mean accumulation ratios for Cmax and AUCtau were 3.00-4.06 and 2.94-4.41, respectively, indicating drug accumulation after multiple doses.
Table 8. Pharmacokinetic parameters after a single subcutaneous injection of AMG 570 in healthy subjects [7]
Dose (mg) | Tmax (day) | Cmax (μg/mL) | AUC0-inf (μg∙day/mL) | t1/2 (day) | CL/F (L/day) |
7 | 5.0 | 0.0864 | 0.928 | 3.21 | 7.88 |
21 | 3.0 | 0.624 | 6.24 | 3.57 | 3.78 |
70 | 3.0 | 4.33 | 51.1 | 3.42 | 1.66 |
140 | 5.0 | 6.05 | 88.6 | 3.79 | 2.37 |
210 | 5.0 | 17.2 | 284 | 4.32 | 0.768 |
420 | 5.0 | 33.5 | 624 | 4.09 | 0.719 |
700 | 5.0 | 58.5 | 1660 | 7.00 | 0.504 |
Antibody-NaV1.7 Inhibitory Peptide Conjugates
The Murray team [9] discovered GpTx-1 and JzTx-V peptides from tarantula venom, which have the effect of inhibiting the voltage-gated sodium channel NaV1.7 and are expected to become novel analgesic drugs. When GpTx-1 was conjugated to an antibody (Conjugate 1), its half-life was extended by 130-fold (80 hours), but its in vitro and in vivo activity was not ideal. JzTx-V peptide is a peptide with higher activity obtained through further research by the team. The team prepared a series of antibody-JzTx-V peptide conjugates and studied the PK and PD properties of the different conjugates. This exploratory research is shared below.
Exploration 1: APC Conjugates with different linker lengths, symmetry, and DAR
First, using the E384C site on the αDNP antibody (non-NaV1.7 targeting, currently lacking effective NaV1.7 antibodies) as the conjugation site, Conjugates 2, 3, and 4 were prepared. Conjugate 2 was symmetrically conjugated with a PEG11 linker, DAR=2; Conjugate 3 was asymmetrically conjugated using an extended PEG linker, DAR=2; Conjugate 4 increased the DAR to 4 based on Conjugate 3. Increasing linker length or asymmetric geometry might promote warhead interaction with the cell membrane. Compared to 2 (IC50=75 nM), the in vitro activity of both 3 (IC50=7.8 nM) and 4 (IC50=2.3 nM) was significantly improved.
In a PK study in mice at 5 mg/kg, the total antibody exposure levels and half-lives of Conjugates 2-4 were much lower than those of the αDNP antibody or the αDNP antibody-linker control group. Their serum concentrations were already lower than the unconjugated control group after 0.5 h and decreased rapidly within the first 24 hours. Although Conjugates 3 and 4 improved in vitro activity compared to 2 by increasing linker length and/or DAR, they correspondingly reduced serum exposure. All conjugates achieved only a 1-fold exposure multiple (serum concentration / hNaV1.7 IC50) within 8 hours, far below the concentration multiple required for in vivo efficacy.
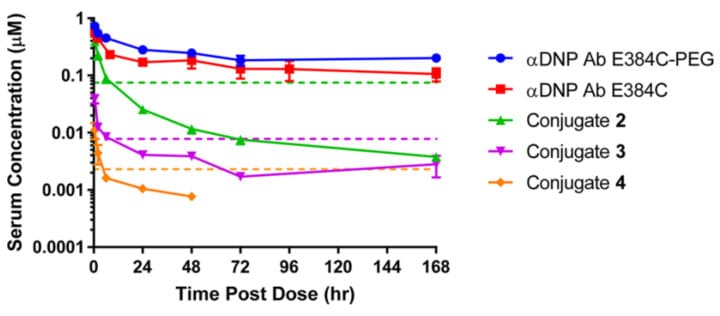
Figure 5. Pharmacokinetic curves for total antibody after IV administration of 5 mg/kg different conjugates and naked antibody in CD-1 mice [9]
To further investigate the reason for the reduced serum exposure of the conjugates, the team used unconjugated antibody, Conjugate 1, and Conjugate 4 derivatized with a 64Cu chelator and studied their biodistribution 20 hours post-dose in mice via PET/CT imaging. The results showed that most of the unconjugated antibody remained in the circulatory system, while Conjugate 4 accumulated extensively in the liver, with its liver accumulation being 4 times that of the unconjugated antibody and Conjugate 1. Therefore, increasing the JzTx-V peptide DAR may promote specific binding and distribution to liver tissue.
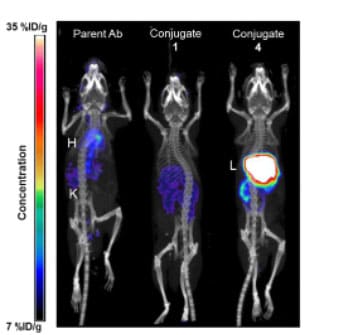
Figure 6. PET/CT imaging 20 h after IV administration of 30-40 mg/kg 64Cu-labeled conjugates and controls in mice [9]
Exploration 2: APC Conjugates with different conjugation sites
Further exploration involved changing the antibody conjugation site to improve the potency of the NaV1.7 inhibitory peptide conjugate. Conjugation sites with higher potency than E384C were screened, such as T487C on the heavy chain and D88C on the light chain, and the corresponding Conjugates 5 and 6 (both DAR=2) were prepared. Compared to Conjugate 2, the activity of Conjugates 5 and 6 increased by approximately 30-fold, suggesting that conjugating the JzTx-V peptide at sites closer to the ends of the IgG scaffold might allow better access to target ion channels on the cell surface. Mouse PK results showed that compared to Conjugate 2, the serum AUC of Conjugates 5 and 6 decreased by 1.9-fold and 2.3-fold, respectively. Apart from the cysteine mutation sites, the chemical composition of these three conjugates was nearly identical, yet they exhibited different activities and PK characteristics. These conjugates maintained serum concentrations above their in vitro IC50 values for nearly 7 days.
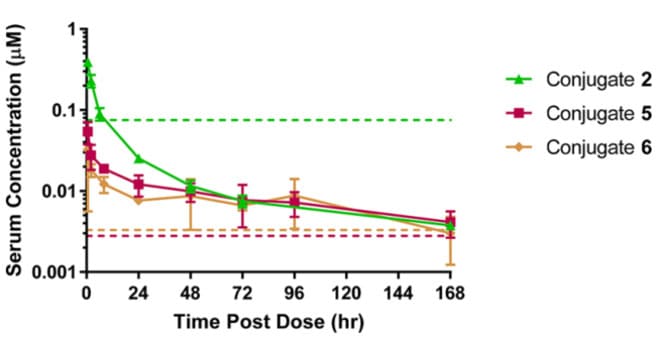
Figure 7. Pharmacokinetic curves for total antibody after IV administration of 5 mg/kg Conjugates 2, 5, and 6 in CD-1 mice [9]
Exploration 3: APC Conjugates with different isoelectric points (pI)
Based on prior experience, the isoelectric point (pI) significantly impacts the pharmacokinetic and tissue distribution of the antibody. Therefore, the researchers hypothesized that reducing the pI of the JzTx-V peptide analog while retaining NaV1.7 potency and selectivity might improve the in vivo PK properties of the conjugate and increase target exposure. Through amino acid sequence adjustments and modifications, the team prepared JzTx-V analogs with net charges of +3, +4, and +2, corresponding to Conjugates 7, 8, and 9 with hNaV1.7 IC50 values of 10.4, 3.1, and 16.4 nM, respectively. Mouse PK results showed an inverse correlation between conjugate exposure (AUC0-last) and increasing net charge (Conjugate 9 (net charge +2) > Conjugate 7 (net charge +3) > Conjugate 8 (net charge +4) > Conjugate 6 (net charge +6)).
Through continuous optimization, using the JzTx-V peptide analog with a net charge of +2 and a glycine-glycine-serine (GGS) linker, Conjugate 10 with high in vitro potency (IC50=1.6 nM) was obtained. Mouse PK studies showed that although the serum exposure of Conjugate 10 was not as high as that of the equally charged Conjugate 9, its in vitro activity was 10 times greater than that of Conjugate 9.
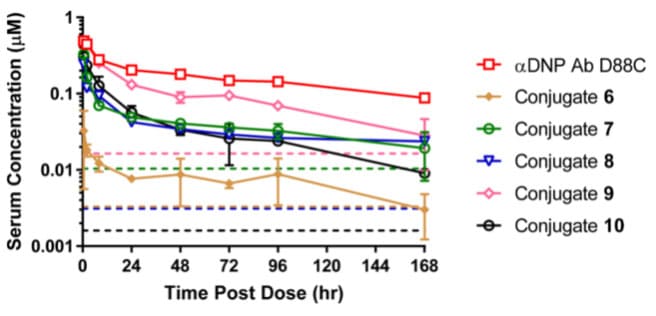
Figure 8. Pharmacokinetic curves for total antibody after IV administration of 5 mg/kg Conjugates 6-9 and naked antibody in CD-1 mice [9]
This research designed a series of antibody-NaV1.7 inhibitory peptide conjugates, exploring the PK and PD properties of the conjugates from multiple perspectives, including linker, DAR, conjugation site, and peptide pI. Although an ideal conjugate molecule has not yet been obtained, these experiences provide valuable references for APC research.
Final Thoughts
APC drugs combine the advantages of antibodies and peptide drugs, enabling benefits such as synergistic effects targeting multiple targets and prolonging the half-life of peptide drugs. As a new drug modality, they represent a novel field in drug research and development. APC drugs are being actively developed in multiple disease areas, both oncological and non-oncological. Encouraging results are also being achieved in pipelines in the preclinical and clinical stages. The endless possibilities brought by different combinations of antibodies, peptides, and linkers in APCs also come with numerous challenges. Compared to traditional monoclonal antibodies and peptide drugs, APCs, as conjugated drugs, have more complex research requirements regarding pharmacokinetics, efficacy, and safety, along with higher R&D difficulty and cost. WuXi AppTec DMPK possesses extensive experience in in vivo and in vitro research on conjugated drugs and can rapidly assist drug developers in efficiently evaluating the ADME characteristics of APC drugs at different research stages, laying a solid foundation for safety and clinical studies.
Authors: Xinmeng Shi, Qian Li, Liping Ma, Jing Jin
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Véniant MM, Lu SC, Atangan L, et al., A GIPR antagonist conjugated to GLP-1 analogues promotes weight loss with improved metabolic parameters in preclinical and phase 1 settings. Nat Metab. 2024 Feb;6(2):290-303.
[2] Petruzzella A, Bruand M, Santamaria-Martínez A, et al., Antibody-peptide conjugates deliver covalent inhibitors blocking oncogenic cathepsins. Nat Chem Biol. 2024 Sep;20(9):1188-1198.
[3] Tian Z, Yu C, Zhang W, et al., Bone-Specific Enhancement of Antibody Therapy for Breast Cancer Metastasis to Bone. ACS Cent Sci. 2022 Mar 23;8(3):312-321.
[4] Lu SC, Chen M, Atangan L, et al., GIPR antagonist antibodies conjugated to GLP-1 peptide are bispecific molecules that decrease weight in obese mice and monkeys. Cell Rep Med. 2021 Apr 30;2(5):100263.
[5] Zhang M, Lee F, Knize A, et al., Development of an ICOSL and BAFF bispecific inhibitor AMG 570 for systemic lupus erythematosus treatment. Clin Exp Rheumatol. 2019 Nov-Dec;37(6):906-914. Epub 2019 Feb 15. PMID: 30789152.
[6] Abuqayyas L, Chen PW, Dos Santos MT, et al., Pharmacokinetics and Pharmacokinetic/Pharmacodynamic Properties of Rozibafusp Alfa, a Bispecific Inhibitor of BAFF and ICOSL: Analyses of Phase I Clinical Trials. Clin Pharmacol Ther. 2023 Aug;114(2):371-380.
[7] Naing A, Thistlethwaite F, De Vries EGE, et al., CX-072 (pacmilimab), a Probody ® PD-L1 inhibitor, in advanced or recurrent solid tumors (PROCLAIM-CX-072): an open-label dose-finding and first-in-human study. J Immunother Cancer. 2021 Jul;9(7):e002447.
[8] Sanborn RE, Hamid O, de Vries EG, et al., CX-072 (pacmilimab), a Probody PD-L1 inhibitor, in combination with ipilimumab in patients with advanced solid tumors (PROCLAIM-CX-072): a first-in-human, dose-finding study. J Immunother Cancer. 2021 Jul;9(7):e002446.
[9] Murray JK, Wu B, Tegley CM, et al., Engineering NaV1.7 Inhibitory JzTx-V Peptides with a Potency and Basicity Profile Suitable for Antibody Conjugation To Enhance Pharmacokinetics. ACS Chem Biol. 2019 Apr 19;14(4):806-818.
[10] Boustany LM, LaPorte SL, Wong L, et al., A Probody T Cell-Engaging Bispecific Antibody Targeting EGFR and CD3 Inhibits Colon Cancer Growth with Limited Toxicity. Cancer Res. 2022 Nov 15;82(22):4288-4298.
[11] Desnoyers LR, Vasiljeva O, Richardson JH, et al., Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Sci Transl Med. 2013 Oct 16;5(207):207ra144.
[12] Camacho RC, You S, D'Aquino KE, et al., Conjugation of a peptide to an antibody engineered with free cysteines dramatically improves half-life and activity. MAbs. 2020 Jan-Dec;12(1):1794687.
[13] Amgen Business Review Meeting. Retrieved February 8, 2022, from http://investors.amgen.com./static-files/6d823d7d-2fd1-405a-8c0e-2aa91bee682
Related Services and Platforms




Stay Connected
Keep up with the latest news and insights.