With an aging population and lifestyle changes such as prolonged use of electronic devices, an increasing number of people are suffering from ocular diseases worldwide. Age-related macular degeneration (AMD) is a leading cause of low vision and blindness in the elderly population[1]. It has become one of the areas with the highest growth potential in the field of ophthalmology. Diabetic retinopathy (DR), as one of the common chronic complications of diabetes, is also a major cause of adult blindness. With the increasing prevalence of diabetes, the number of people affected by DR is also increasing gradually[2]. Additionally, immune-related ophthalmopathies such as uveitis and thyroid ophthalmopathy have become one of the main diseases affecting the vision health of young people. The growing demand for treating ocular diseases is driving the development and launch of better treatment options. Currently, the innovative ophthalmic drug market is in a phase of steady and rapid growth. According to Frost & Sullivan's Research Report on the Development Status and Future Trends of the Ophthalmic Drug Market, the global innovative ophthalmic drug market is projected to reach $46.4 billion in 2025 and $73.9 billion in 2030. Based on approved drugs and our accumulated R&D experience, this article summarizes the current landscape of ophthalmic protein drugs and the design of preclinical pharmacokinetic experiments, aiming to provide references for the R&D of such drugs.
Summary of Ocular Therapeutic Protein Drugs Approved by FDA/NMPA/PMDA by 2025
Since the approval of ranibizumab by the FDA in 2006, research on ocular therapeutic protein drugs has become a hotspot, especially in the treatment fields of fundus vascular diseases and immune-related eye diseases. To date, approximately 11 protein-based ophthalmic drugs have been approved globally, including different molecular formats such as antigen-binding fragments (Fab), monoclonal antibodies (mAbs), fusion proteins, single-chain variable fragments (scFv), and bispecific antibodies (Figure 1). Related indications include wet age-related macular degeneration (wAMD), diabetic macular edema (DME), neuromyelitis optica spectrum disorder (NMOSD), and thyroid ophthalmopathy. Table 1 summarizes information on the targets, skeleton structures, administration routes, administration frequency, and indications of currently approved ocular therapeutic protein drugs.
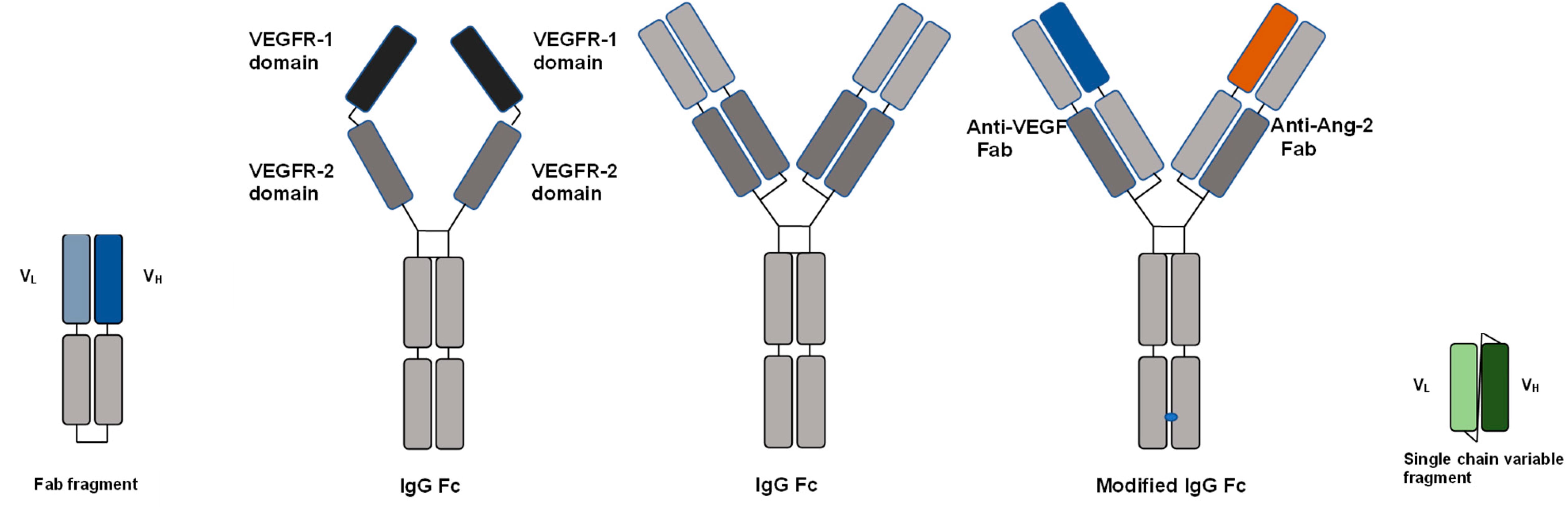
Figure 1. Molecular formats of ocular therapeutic protein drugs[3]
Within the ophthalmic drug market, drugs for fundus vascular diseases hold the largest share, predominantly those targeting vascular endothelial growth factor (VEGF). VEGF is a glycoprotein composed of disulfide-linked homodimers, widely distributed in human tissues and organs such as the brain, eye, kidney, and liver. Among them, periretinal cells, endothelial cells, pigment epithelial cells, and ganglion cells in the eye can express VEGF, which plays an important role in maintaining vascular integrity. The VEGF family includes five members: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PLGF). Among them, VEGF-A is the most potent and is closely associated with neovascularization and increased vascular permeability[4]. Overexpression of VEGF leads to abnormal neovascularization, causing retinal leakage and edema in ocular diseases, severely impairing patients' vision. The currently approved drugs aflibercept, conbercept, ranibizumab, brolucizumab, and faricimab all target VEGF (Figure 2a)[5]. Besides the VEGF target, adalimumab inhibits inflammatory factors like TNF-α, induces an increase in Treg cells, and repairs the blood-retinal barrier. Eculizumab inhibits C5 complement-mediated inflammatory responses. Inebilizumab, rituximab, and satralizumab act on various stages of B-cell maturation and differentiation, targeting CD19, CD20, and IL-6 receptor, respectively. They inhibit the production of antibodies, thereby protecting the aquaporin-4 (AQP4) channel on the surface of optic nerve cells from attack (Figure 2b, 2c)[6,7]. Teprotumumab targets the insulin-like growth factor-1 receptor (IGF-1R), widely expressed in orbital tissues, thereby interfering with the signal transduction pathway mediated by the autoantigen thyroid-stimulating hormone receptor and hindering disease progression[8].
Table 1. Approved ocular therapeutic protein drugs*
Generic name | Brand name | Target | Drug format (Molecular weight) | Skeleton structure | Administration Route (Dose frequency) | Indication | Company | First approval (Year, Country) |
ranibizumab | Lucentis | VEGF-A | Fab (48 kDa) | Fab, no Fc region | IVT (q4w) | wAMD, DME, RVO, CNV, DR, ROP | Novartis/Roche | 2006, USA |
aflibercept | Eylea | VEGF | Fusion protein (115 kDa) | Fusion with the human IgG1 Fc region | IVT (q8w initially, then q8w or q12w or q16w) | wAMD, DME, RVO, DR, ROP | Regeneron/Bayer | 2011, USA |
conbercept | Lumitor (朗沐) | VEGF | Fusion protein (~142 kDa) | Fusion with the human IgG1 Fc region | IVT (monthly for first 3 months, then q3 months) | wAMD, DME, RVO | Kanghong Pharma | 2013, China |
adalimumab | Humira | TNF-α | mAb (~148 kDa) | Humanized IgG1 | SC (qw) | Non-infectious uveitis | AbbVie/Eisai | 2016, USA |
eculizumab | Soliris | C5 | mAb (~148 kDa) | Humanized IgG2/4 | IV (qw) | NMOSD | AstraZeneca | 2019, USA |
brolucizumab | Beovu | VEGF-A | scFv (26 kDa) | Humanized single-chain antibody fragment | IVT (q4-6w for first 3-5 injections, then q8-12w) | wAMD, DME | Novartis | 2019, USA |
inebilizumab | Uplizna | CD19 | mAb (~149 kDa) | Humanized IgG1 | IV (q2w for first 2 doses, then q6 months) | NMOSD | Viela Bio/Horizon | 2020, USA |
satralizumab | Enspryng | IL-6R | mAb (~143 kDa) | Humanized IgG2 | SC (q2w for first 3 doses, then q4 weeks) | NMOSD | Roche/Chugai | 2020, Japan |
teprotumumab | Tepezza | IGF-1R | mAb (~148 kDa) | Humanized IgG1 | IV (q3w) | Thyroid eye disease | Horizon/Amgen | 2020, USA |
faricimab | Vabysmo | VEGF-A/Ang2 | Bispecific antibody (146 kDa) | Human IgG1, engineered Fc unable to bind FcγR, VEGF-binding Fab identical to ranibizumab | IVT (q4w for at least 4 doses, up to q16w) | wAMD, DME, RVO | Roche/Genentech | 2022, USA |
rituximab | Ristova (for NMOSD) | CD20 | mAb (145 kDa) | Chimeric (human IgG1 Fc, murine variable region) | IV (qw or q2w) | NMOSD | Roche/Biogen | 2022, Japan |
* Biosimilars are not included.
*Abbreviations: VEGF: vascular endothelial growth factor; PLGF: placental growth factor; VEGFR: vascular endothelial growth factor receptor; ANG: angiopoietin; IGF-1R: insulin-like growth factor 1 receptor; SC: subcutaneous injection; IV: intravenous injection; IVT: intravitreal injection; qw: once weekly; q2w: once every 2 weeks; q3w: once every 3 weeks; q4w: once every 4 weeks; q8w: once every 8 weeks; q12w: once every 12 weeks; q16w: once every 16 weeks; wAMD: wet age-related macular degeneration; DME: diabetic macular edema; DR: diabetic retinopathy; CNV: choroidal neovascularization; RVO: retinal vein occlusion; ROP: retinopathy of prematurity; NMOSD: neuromyelitis optica spectrum disorders.*
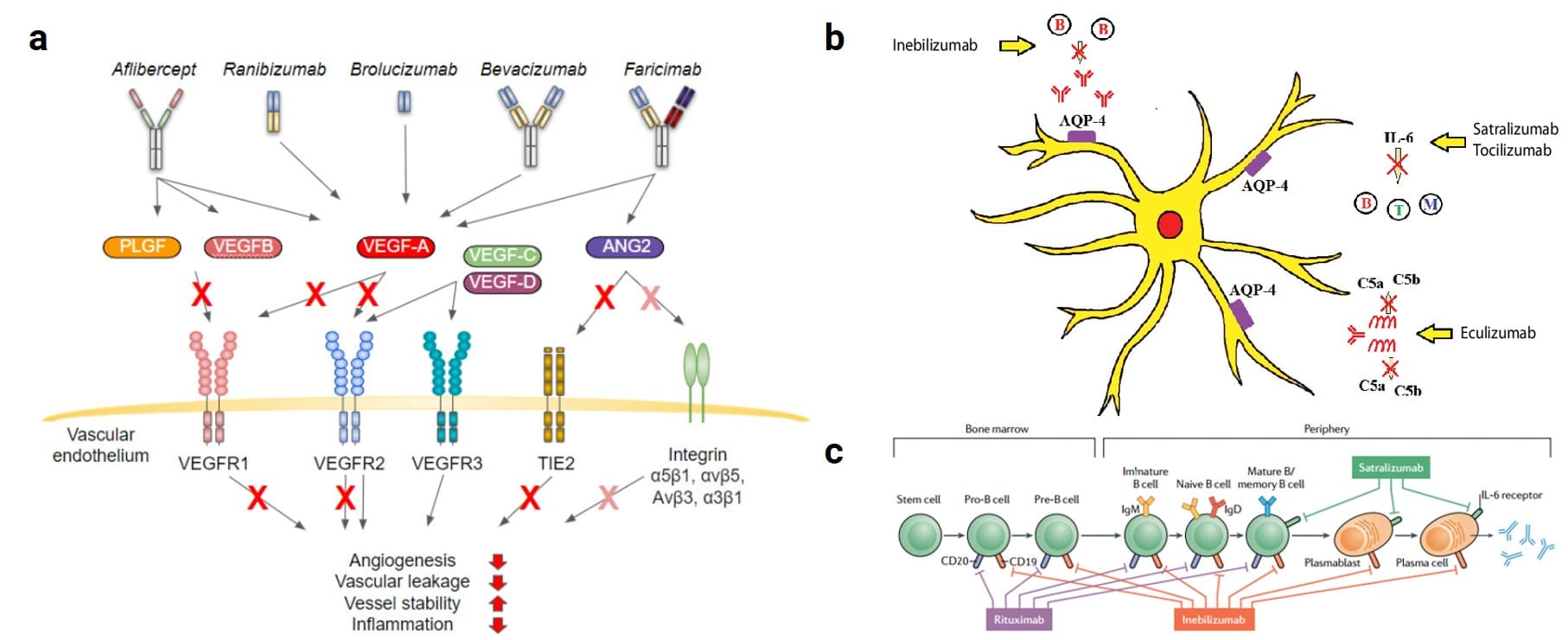
Figure 2. (a) Mechanism of action of anti-VEGF therapy in ocular diseases (bevacizumab is used off-label)[5]; (b) Mechanism of inebilizumab, satralizumab, and eculizumab in protecting nerve cells[6]; (c) Rituximab, inebilizumab, and satralizumab acting on different stages of B-cell development, respectively[7].
Preclinical Pharmacokinetic Considerations for Ophthalmic Protein Drugs
The design of preclinical pharmacokinetic (PK) experiments for ophthalmic protein drugs is closely related to their administration routes.
Systemically Administered Drugs
For drugs administered via systemic routes, the preclinical PK study design can refer to conventional methods for protein drugs. PK studies are generally conducted in relevant animal species, usually selecting two or more dose levels. If the drug is intended for subcutaneous (SC) administration clinically, it is necessary to carry out animal PK studies of IV and SC. As shown in Table 2, adalimumab, eculizumab, inebilizumab, satralizumab, and teprotumumab all conducted animal PK studies only via systemic administration routes.
Intravitreally (IVT) Administered Drugs
If the clinical route of administration is intravitreal (IVT) injection, it is suggested to carry out the serum PK and eye tissue distribution in animals after IVT administration.. Although IVT injection is invasive, it allows for high drug concentrations in the vitreous humor, and it is an effective route for various fundus diseases. Currently approved protein drugs for IVT administration include ranibizumab, aflibercept, conbercept, brolucizumab, and faricimab (Table 3).
Table 2. Preclinical PK study design for five systemically administered ocular protein drugs*
Generic name (Reference) | Clinical route | Preclinical PK study | Analyte | Toxicology species |
adalimumab[9] | SC | Single-dose PK study in Cynomolgus monkeys after IV and SC administration | Serum PK and ADA detection | Cynomolgus monkey, rat, mouse |
Single-dose PK study in Cynomolgus monkeys with a single IV dosage and 3 SC dosages | Serum PK and ADA detection | |||
Single Mouse PK study after IV administration | Serum PK | |||
eculizumab[10] | IV | Single-dose PK study in C5-deficient mice after IV and SC administration | Serum PK and serum PD markers | Mouse |
inebilizumab[11] | IV | Single PK study in huCD19 transgenic mice after IV administration with two dose levels (combined with tox study) | Serum PK | huCD19 transgenic mouse |
satralizumab[12] | SC | Single PK study in Cynomolgus monkeys with 4 dosages (2 IV, 2 SC) | Serum PK, IL-6 levels, and ADA detection | Cynomolgus monkey |
teprotumumab[13] | IV | Single PK study in male rats with 3 IV dosages; | Serum PK, ADA detection | Cynomolgus monkey |
PK/PD study in cynomolgus monkeys | Serum PK, ADA, and PD (IGF-1R) markers |
*Rituximab is approved for multiple indications; specific preclinical PK studies for its NMOSD approval in Japan were not identified. *Abbreviations: PK: pharmacokinetics; ADA: anti-drug antibody; PD: pharmacodynamics; C5: complement component 5; IL-6: interleukin-6.*
Table 3. Preclinical PK study design for four IVT-administered ocular protein drugs*
Generic name (Reference) | Clinical route | Preclinical PK study design | Analyte | Toxicology species |
Ranibizumab[14] | IVT | PK study in male NZW rabbits with two dosages after IVT administration to both eyes | Drug concentration in serum | Cynomolgus monkey, NZW rabbit |
PK study in male NZW rabbits with two dosages after IVT administration into both eyes | Drug concentration in vitreous, aqueous humor, serum; ADA in vitreous and serum | |||
Ocular PK studies in male NZW rabbits after IVT to both eyes of 125I-labeled antibody | Drug distribution in the eyeballs (3 time points) | |||
Single-dose PK study in NZW rabbits to compare IVT, subconjunctival injection, and anterior chamber injection (pilot study) | Drug concentration in vitreous, aqueous humor, retina (2 time points each) | |||
Single-dose PK study in NZW rabbits to compare IVT, subconjunctival injection, and anterior chamber injection | Drug concentration in vitreous, aqueous humor, retina, serum (6 time points each) | |||
Single dose PK study in Cynomolgus monkeys after IVT and IV administration with two dose levels each | Drug concentration in vitreous humor, retina, aqueous humor, serum | |||
Aflibercept[15] | IVT | Single-dose PK study in pigmented rabbits after IVT administration | Drug concentration in vitreous, retina, choroid, serum | SD rat, Cynomolgus monkey |
Single-dose PK study in Cynomolgus monkeys after IV (single group) and SC (four groups) administration | Drug concentration in serum | |||
Tissue distribution in female SD rats after IV administration of 125I-labeled aflibercept | Radioactivity in major tissues/organs | |||
Single PK studies in nephrectomized vs. sham-operated SD rats after IV administration | Assess renal contribution to clearance. | |||
Brolucizumab[16] | IVT | Single dose PK in Cynomolgus monkeys (both sexes) via IV administration | Serum PK and ADA detection | Cynomolgus monkey |
Single serum PK and ocular PK in NZW rabbits after IVT administration to a single eye | 1. Drug concentration in vitreous humor, retina, choroid, aqueous humor, serum; ADA not assessed. | |||
Ocular PK in Cynomolgus monkeys after single IVT administration to a single eye (pilot study) | Drug concentration in central retina, peripheral retina, vitreous humor, aqueous humor, central choroid, peripheral choroid, serum (7 time points); serum ADA. | |||
Ocular PK in Cynomolgus monkeys after single IVT administration (dose, injection volume, vehicle evaluation) | Drug concentration in central retina, peripheral retina, vitreous humor, aqueous humor, central choroid, peripheral choroid, serum (all samples); serum ADA. | |||
Faricimab[17] | IVT | Serum PK in NZW rabbits with two dose levels of IV administration | Serum PK and ADA | Cynomolgus monkey |
Serum PK and ocular PK in Cynomolgus monkeys after a single IV and IVT dose | ocular PK (Vitreous humor and aqueous humor), serum PK; serum ADA | |||
Serum PK and ocular PK in pigmented rabbits after a single dose of IVT | ocular PK (Vitreous humor and aqueous humor), serum PK, serum ADA | |||
Serum PK and ocular PK in Cynomolgus monkeys after single IV and IVT administration | ocular PK (aqueous humor), serum PK, and ADA in aqueous humor and serum |
*Preclinical PK study design for the concept was not identified.
Animal Species Selection in Pharmacokinetic Studies
Rabbits are generally chosen as one of the animal species for IVT PK studies primarily because the anatomy and physiology of the rabbit eye are similar to the human eye. Additionally, rabbits have large eyes, facilitating IVT injection operation and observations, and offer advantages such as low cost and easy access[18]. In the regulatory submissions of the four approved IVT-administered drugs, rabbits were sacrificed to collect major ocular tissues, and drug concentrations were analyzed after IVT administration. It is important to note that while rabbits can be a suitable species for ocular tissue distribution studies, they may not always be a relevant species for toxicology studies. For example, for brolucizumab, target binding affinity indicated that the rabbit was not a relevant species. Consequently, only cynomolgus monkeys were selected as the relevant species for toxicology studies in brolucizumab's preclinical development. Considering the physiological and anatomical advantages of both rabbits and monkeys, drug distribution is usually conducted in both rabbits and monkeys for ophthalmic protein drugs.
Conventional New Zealand White (NZW) rabbits are albino animals lacking intraocular melanin, unlike the human eye, which is rich in melanin. Melanin deficiency may affect drug distribution within the eye (Further reading: What is Melanin Binding and How to Study It In Vitro?). Pigmented rabbits are utilized for ocular PK studies after IVT administration for aflibercept and faricimab. Furthermore, rabbits are highly prone to developing anti-drug antibodies (ADA), which can significantly impact systemic drug exposure, making them potentially unsuitable for toxicology studies. For instance, rabbits were used in PK studies of faricimab, but weren’t employed as a toxicology species due to the high incidence of ADA.
PK Modeling for Ophthalmic Protein Drugs
Absorption, distribution, and elimination data in animal ocular tissues after IVT administration can be used to predict drug concentrations in human ocular tissues. During the development of ranibizumab, researchers established a 6-compartment model based on PK data from rabbits and monkeys following both IV and IVT administration (Figure 3)[14]. For the IV route, drug concentration in serum was predicted using a 2-compartment model. For the IVT route, the eye was divided into 4 compartments: central vitreous (Compartment 3), vitreous body (Compartment 4), anterior chamber (Compartment 5), and retina (Compartment 6). The drug ratio in the vitreous to retina was relatively similar between rabbits and monkeys after IVT administration of ranibizumab. Serum concentrations were more than 1000-fold lower than vitreous concentrations, and tissue and serum concentrations showed dose-dependency across different doses. This model proved effective in predicting drug concentrations in the retina and serum of rabbits and monkeys at other doses, providing strong support for predicting human ocular PK. Currently, several different compartment models for IVT administration have been reported in the literature[19], allowing for the selection or development of an appropriate PK model based on the specific characteristics of the drug to predict human ocular PK.
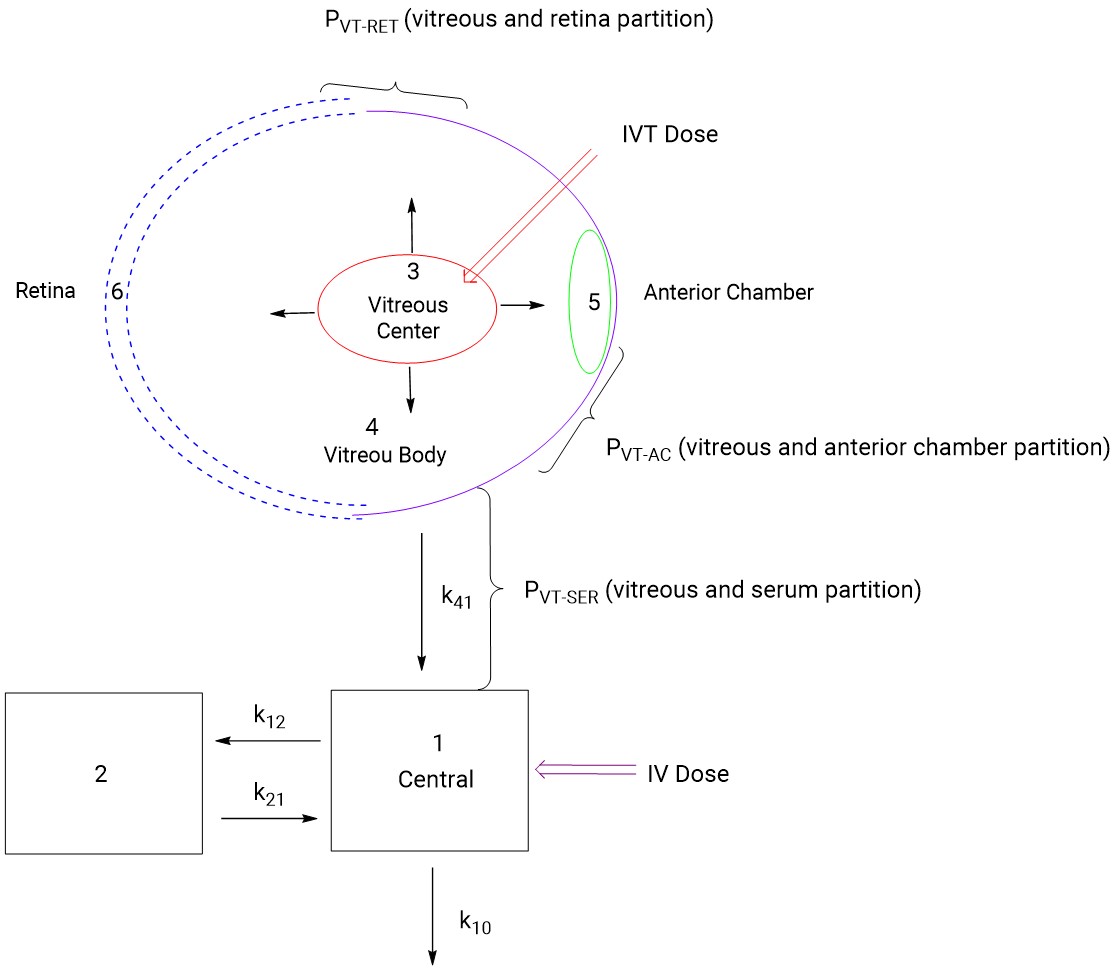
Figure 3. Compartmental model for ocular pharmacokinetics of ranibizumab[14]
Regulatory Considerations
In September 2020, NMPA issued Technical Guidelines for Clinical Research of Age-Related Macular Degeneration Therapeutic Drugs[20]. It provides the following requirements and recommendations for the pharmacokinetic study of ophthalmic drugs: For drugs administered locally to the eye (e.g., intravitreal injection), both local exposure and systemic exposure should be evaluated. For drugs with systemic exposure or administered via systemic routes, conventional systemic pharmacokinetic research methods can be followed to provide data on systemic exposure, drug metabolism, and elimination. For drugs that are primarily distributed locally in the eye and act locally, it is recommended to provide relevant animal data on local ocular pharmacokinetics, including distribution, metabolism, and elimination of the drug in sites such as the aqueous humor and vitreous humor. The application of new methods and technologies is encouraged, including radiolabeled molecular imaging techniques and pharmacokinetic modeling/simulation technologies.
Outlook and WuXi AppTec DMPK Platforms
Current mainstream ocular PK experiments require the use of a significant number of animals to obtain drug concentration data from various ocular tissues. Considering the 3R principles (Replacement, Reduction, Refinement) for animal use, techniques such as quantitative whole-body autoradiography (QWBA) and imaging technologies can be employed to substantially reduce the number of animals used. New technologies continue to emerge, such as ocular microdialysis, which allows for continuous sampling and analysis of drug concentrations after intravitreal administration[21]. WuXi AppTec DMPK has established a preclinical animal ocular PK evaluation platform, proficient in performing various local ocular administrations in preclinical small and large animals, collecting samples from various ocular tissues, and quantifying drug concentrations. Our QWBA platform can be used to evaluate the distribution of ophthalmic drugs in various ocular tissues of preclinical animals, providing support for the R&D of clients' new drugs.
Authors: Qigan Cheng, Lijuan Hou, Jianping Sun, Huan Liu, Jing Jin
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Rein DB, Wittenborn JS, Burke-Conte Z, Gulia R, Robalik T, Ehrlich JR, Lundeen EA, Flaxman AD. Prevalence of Age-Related Macular Degeneration in the US in 2019. JAMA Ophthalmol. 2022 Dec 1;140(12):1202-1208.
[2] Liu Han, Fang Yanhong, Chen Jian. General Practice Management of Diabetic Retinopathy. Eye Science. 2023 Apr;38(4):350-359.
[3] Tatsumi T. Current Treatments for Diabetic Macular Edema. Int J Mol Sci. 2023 May 31;24(11):9591.
[4] Pożarowska D, Pożarowski P. The era of anti-vascular endothelial growth factor (VEGF) drugs in ophthalmology, VEGF and anti-VEGF therapy. Cent Eur J Immunol. 2016;41(3):311-316.
[5] Heloterä H, Kaarniranta K. A Linkage between Angiogenesis and Inflammation in Neovascular Age-Related Macular Degeneration. Cells. 2022 Nov 1;11(21):3453.
[6] Selmaj K, Selmaj I. Novel emerging treatments for NMOSD. Neurol Neurochir Pol. 2019;53(5):317-326.
[7] Pittock SJ, Zekeridou A, Weinshenker BG. Hope for patients with neuromyelitis optica spectrum disorders - from mechanisms to trials. Nat Rev Neurol. 2021 Dec;17(12):759-773.
[8] Jain AP, Jaru-Ampornpan P, Douglas RS. Thyroid eye disease: Redefining its management-A review. Clin Exp Ophthalmol. 2021 Mar;49(2):203-211.
[9] http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/BLA_125057_S000_HUMIRA_PHARMR.PDF
[10] http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/125166s0000_PharmR.pdf
[11] http://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/761142Orig1s000PharmR.pdf
[12] http://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/761149Orig1s000PharmR.pdf
[13] http://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761143Orig1s000PharmR.pdf
[14] http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/125156s0000_Lucentis_PharmR.pdf
[15] http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/125387Orig1s000PharmR.pdf
[16] http://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761125Orig1s000PharmR.pdf
[17] http://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761235Orig1s000PharmR.pdf
[18] Fu Shujun, Yu Bing, Liao Qin, Sun Tao. Research progress and key considerations for non-clinical studies of intravitreally administered drugs. Acta Pharmaceutica Sinica. 2023, 58(4): 815−825.
[19] Agrahari V, Mandal A, Agrahari V, Trinh HM, Joseph M, Ray A, Hadji H, Mitra R, Pal D, Mitra AK. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016 Dec;6(6):735-754.
[20] Center for Drug Evaluation, NMPA. Technical Guideline for Clinical Research of Age-Related Macular Degeneration Therapeutic Drugs. September 2020.
[21] Boddu SH, Gunda S, Earla R, et al. Ocular microdialysis: a continuous sampling technique to study pharmacokinetics and pharmacodynamics in the eye [J]. Bioanalysis, 2010, 2: 487-507.
Stay Connected
Keep up with the latest news and insights.