In innovative drug development, biomarkers, objective indicators of physiological and pathological states as well as drug responses, have become indispensable tools for guiding decision-making. Their application extends across the entire drug development lifecycle: from early-stage target screening and mechanism validation, to patient stratification and pharmacodynamic evaluation in clinical trials, and further to companion diagnostics and safety monitoring after market approval. Developing precise biomarker analysis strategies not only deepens the understanding of a drug’s mechanism of action but also enhances clinical trial design and shortens regulatory review timelines. This article reviews mainstream biomarker analysis platforms, examines their advantages and limitations, and highlights the "fit-for-purpose" validation principle to offer fresh perspectives on optimizing biomarker strategies and accelerating drug R&D.
What Is a Biomarker
A biomarker is a biological indicator, such as an endogenous protein, nucleic acids, or metabolite, which can be measured objectively, accurately, and reproducibly. Biomarkers provide insights into physiological and pathological states and can characterize specific biological responses to drug intervention. They hold significant value across multiple domains, including disease diagnosis, prognosis assessment, and drug development. According to the definition jointly issued by the FDA and NIH in 2016 in Biomarkers, Endpoints, and other Tools (BEST), biomarkers are classified into seven types [1, 2]: diagnostic, monitoring, pharmacodynamic/response, predictive, prognostic, safety, and susceptibility/risk (see Figure 1).
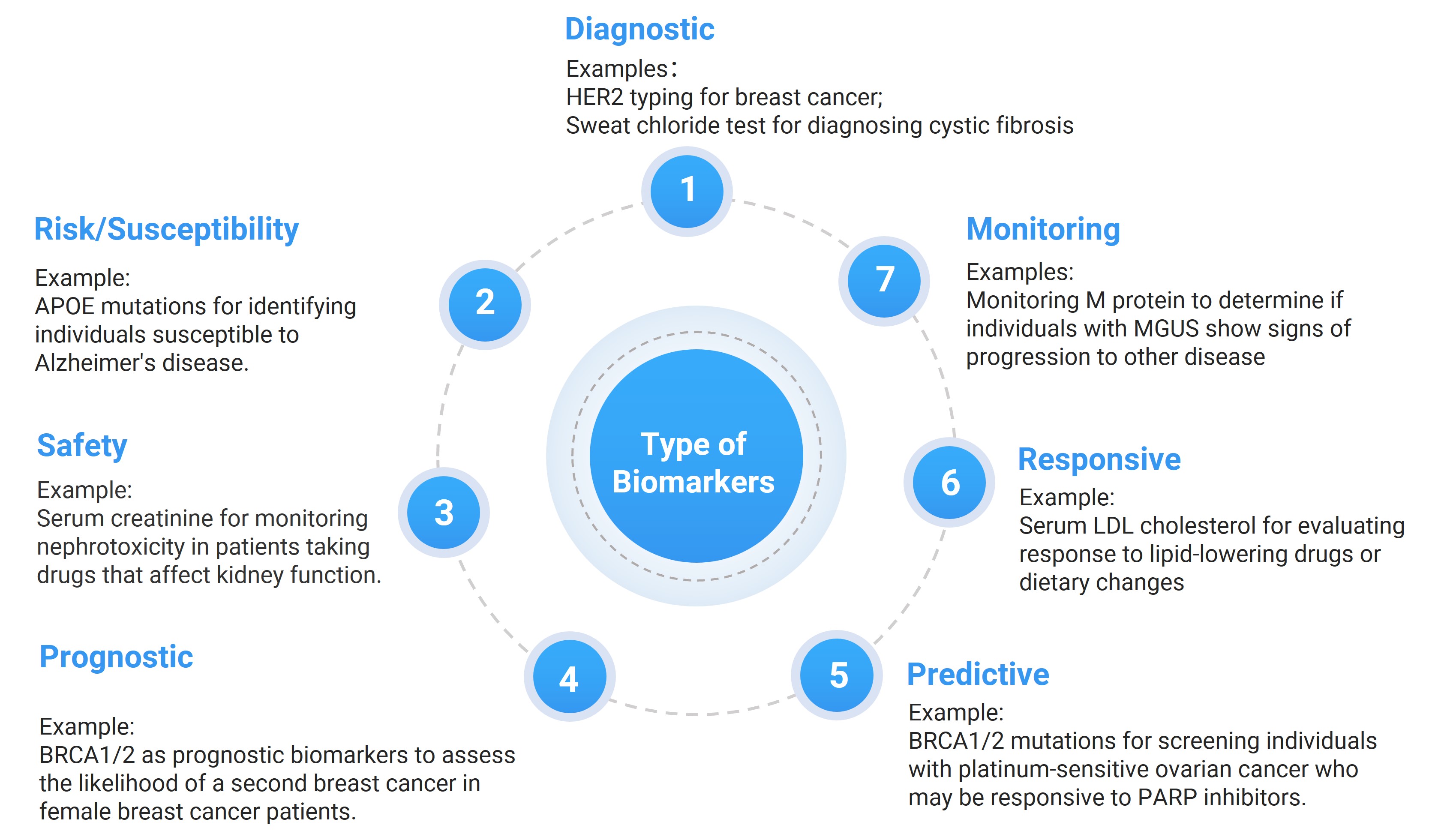
Figure 1. The seven types of biomarkers
Why Biomarker Testing Matters in Drug Development
The detection of biomarkers plays a critical, decision-supporting role throughout drug discovery, development, and registration. In the discovery phase, biomarkers are employed for target screening, elucidating mechanisms of action, and generating key data for proof-of-concept studies. During preclinical research, they provide essential guidance for dose selection and safety evaluation. In the clinical stage, biomarkers support disease diagnosis and stratification, patient enrollment, dose optimization, pharmacokinetic studies, and assessments of safety and efficacy. At the stage of drug approval and commercialization, their applications further expand to companion diagnostic development, continuous monitoring of safety and efficacy, and strengthened quality control.
Establishing robust biomarker strategies not only deepens the understanding of a drug's mechanism of action but also improves patient selection in clinical trials, enables real-time monitoring and prediction of potential toxicity, and provides a scientific basis for both drug development and regulatory decision-making. Collectively, these applications enhance the efficiency and sustainability of drug development, improve drug quality and safety, and accelerate the approval and market entry of new drugs[2, 3].
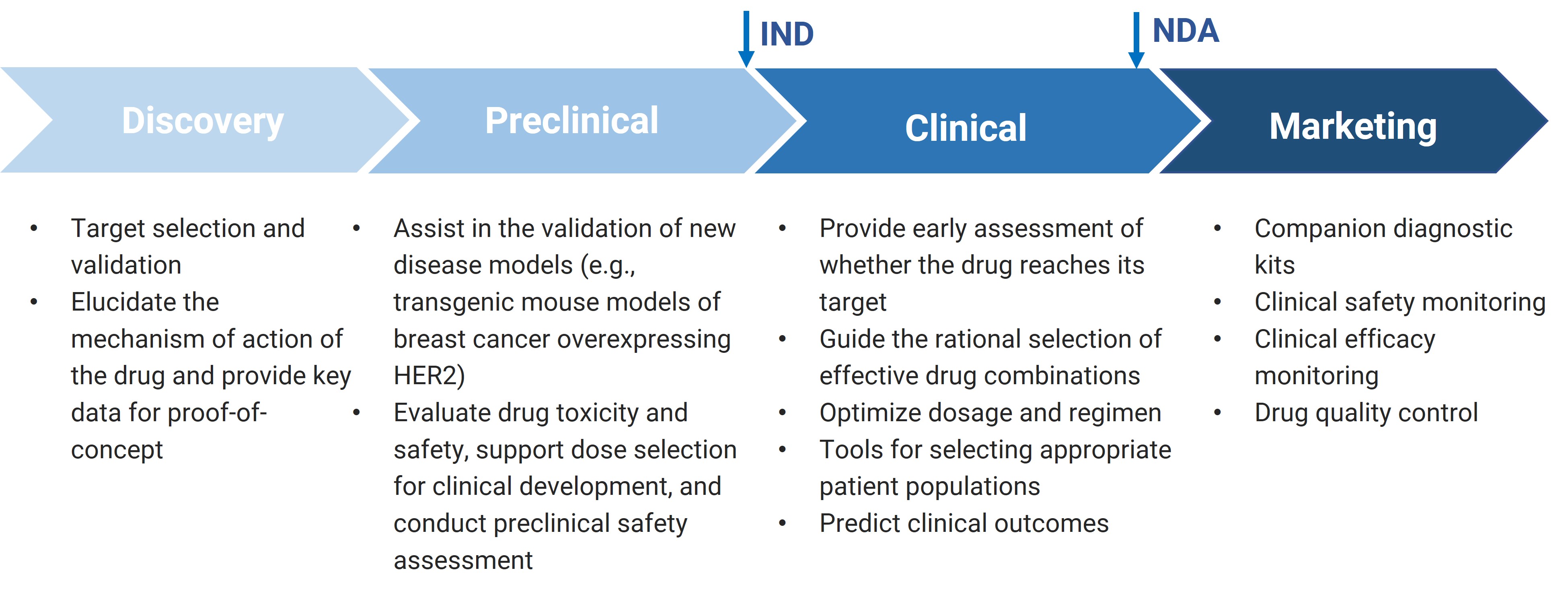
Figure 2. The key role of biomarkers in various stages of new drug development
Analytical Platforms for Biomarker Testing
Biomarkers are highly diverse, ranging from small molecules and proteins to cells, nucleic acids, and tissue sections. Their testing requirements vary considerably across different stages and areas of drug development. Because no single bioanalytical platform can fully meet all biomarker analysis needs, it is often necessary to integrate multiple bioanalytical platforms. Each platform offers unique strengths and faces certain limitations, making a combined approach essential for comprehensive biomarker testing, which varies significantly across different drug development areas[4].
Based on detection principles, commonly used biomarker testing platforms can be primarily classified into three main categories:
Analytical platforms based on liquid chromatography-mass spectrometry (LC-MS/MS);
Analytical platforms based on immunoassays;
Testing platforms based on molecular biology techniques.
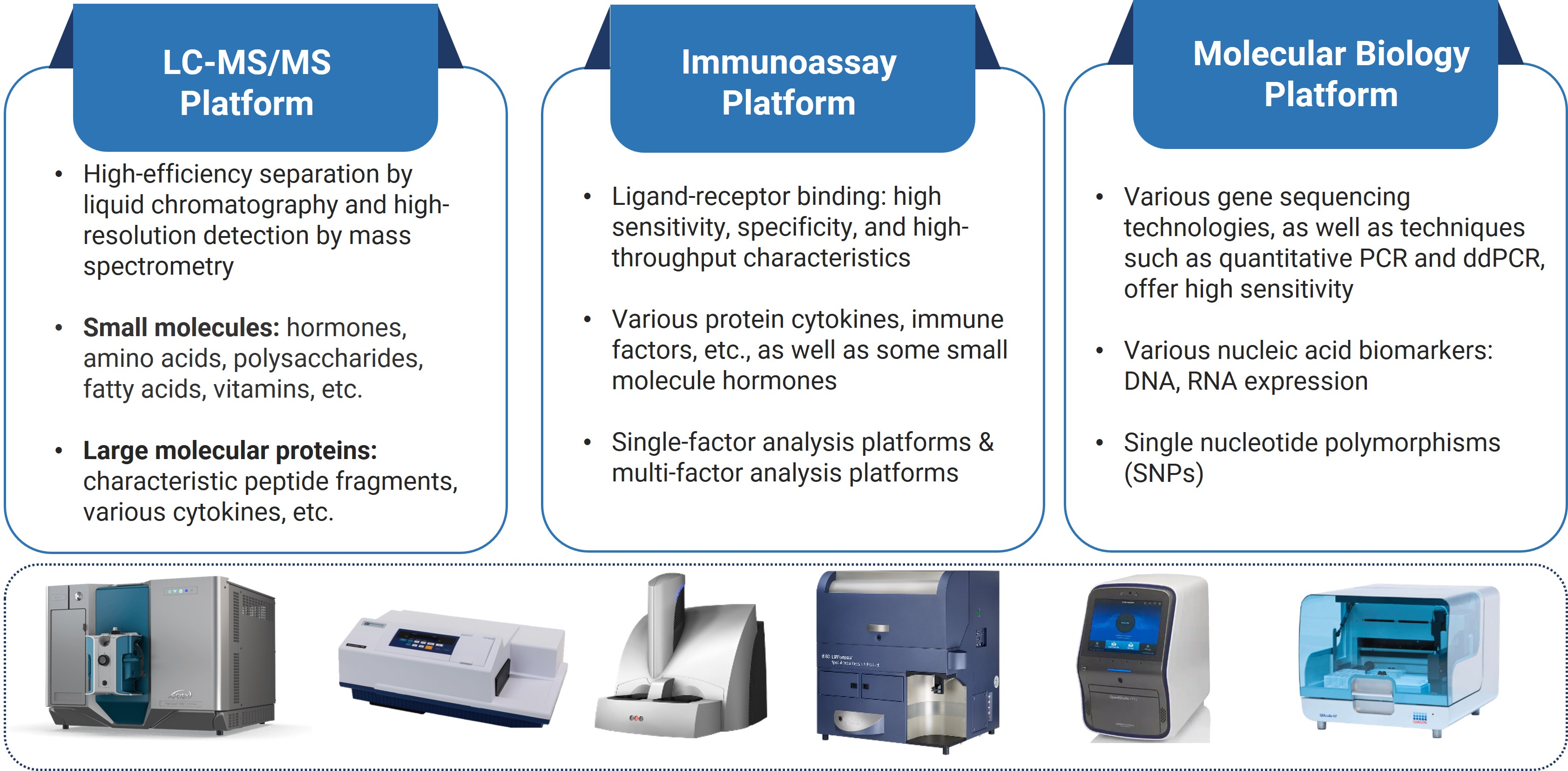
Figure 3. Three mainstream biomarker analytical platforms
Liquid Chromatography-Mass Spectrometry (LC-MS) Based Biomarker Analysis
LC-MS technology combines the high separation efficiency of liquid chromatography with the high-sensitivity and high-resolution detection characteristics of mass spectrometry, delivering strong analytical specificity. It is particularly suitable for the precise quantitative analysis of various biomarkers in complex biological matrices, such as hormones, amino acids, polysaccharides, fatty acids, and vitamins. The advancement of hybrid LC-MS/MS technology (i.e., immunocapture-LC-MS) has demonstrated significant advantages in protein biomarker detection. This approach combines immunocapture with mass spectrometry: specific reagents are first used to enrich the target protein, which is then digested into signature peptides, followed by highly selective and sensitive detection through LC-MS/MS. In certain applications, LC-MS platforms can even deliver greater sensitivity and accuracy than traditional immunoassays, making them a powerful alternative for protein biomarker analysis. [5, 6]
Immunoassay-Based Biomarker Analysis
Ligand binding assays (LBA) are one of the most widely used biomarker testing platforms. Due to their high sensitivity, high specificity, and high throughput, they have become a core detection method.
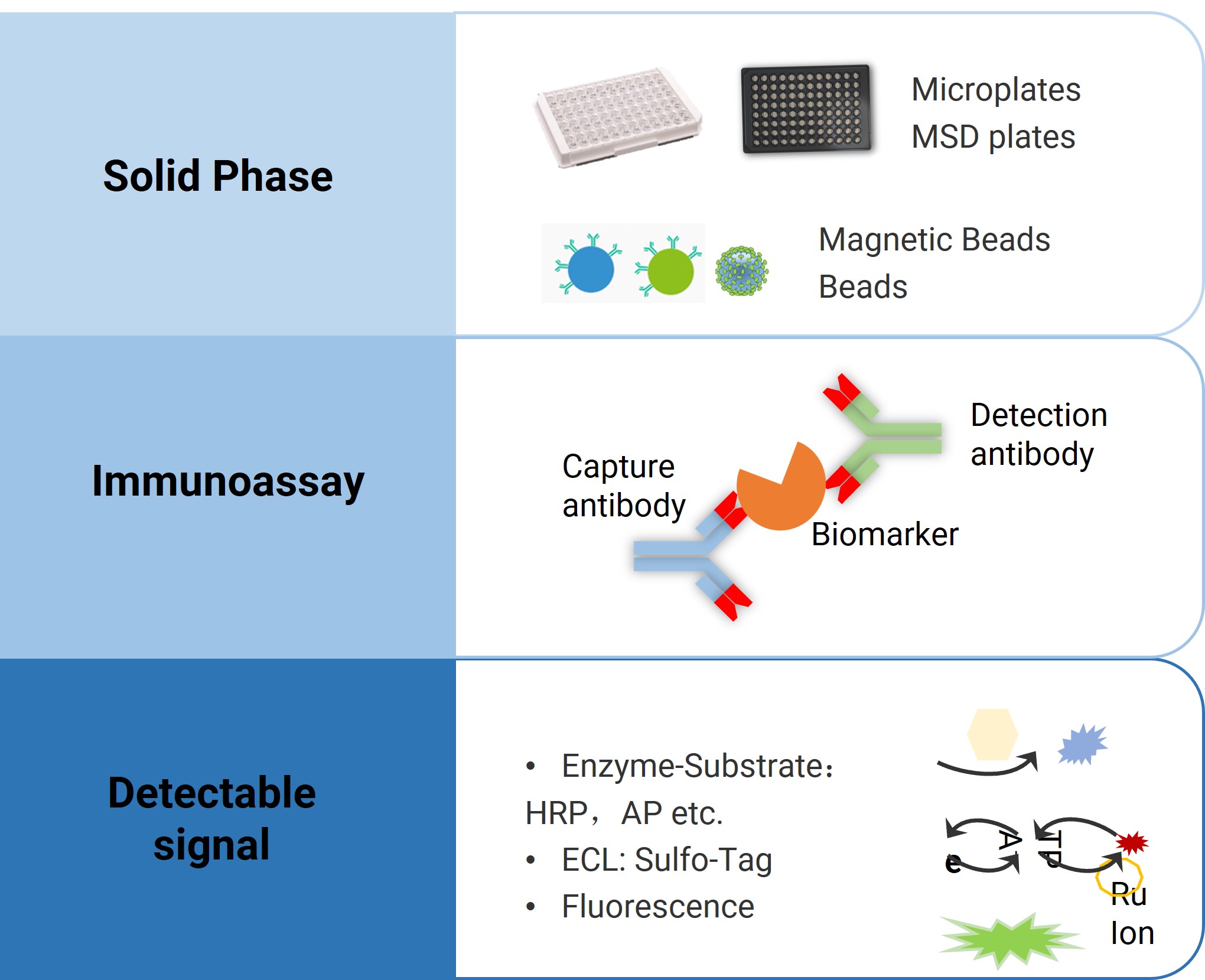
Figure 4. Immunoassay-based biomarker analysis platform
Based on differences in analytic technology and luminescence principles, commonly used immunoassay platforms currently include:
Enzyme-Linked Immunosorbent Assay (ELISA)
Electrochemiluminescence (ECL)
Bead-based platforms like LuminexFlow Cytometry(CBA)
Enzyme-Linked Immunospot (ELISpot)
Single Molecule Array technology (Simoa)
Microfluidic chip-based platforms like GyroLab, etc.
These platforms each have advantages in terms of sensitivity, assay range, sample volume requirement, and detection throughput. In practical applications, it is necessary to comprehensively consider factors such as target molecule type, required detection sensitivity, sample volume, multiplexing capability, and instrument availability to select the most suitable testing platform[7, 8].
For example, ELISA is often selected as a routine biomarker analytical platform because of its simplicity and low cost; MSD, Luminex, and CBA technologies are better suited for high-throughput, multi-factor detection, while Simoa, offering ultra-high sensitivity, is particularly valuable for measuring low-abundance targets such as biomarkers of neurodegenerative diseases. For functional analysis, ELISpot enables the detection of cellular activities like cytokine secretion activity; whereas flow cytometry is widely applied in immunophenotyping and the assessment of certain intracellular markers. For small-volume samples, such as cerebrospinal fluid (CSF), the GyroLab platform provides an effective solution. In practice, the choice of an immunoassay platform should also take into account practical factors, such as instrument availability and accessibility.
In addition to the quantitative immunoassay techniques described above, semi-quantitative analysis techniques also play an important role in biomarker testing. Western blot is widely applied for verifying protein expression levels, confirming molecular weights, studying post-translational modifications, and detecting insoluble proteins in a semi-quantitative manner. Immunohistochemistry (IHC), in addition to offering semi-quantitative analysis, provides valuable spatial information on protein distribution within tissues. When combined with histopathological features, IHC plays an irreplaceable role in pathological diagnosis and mechanism studies. It is especially important in research on the tissue microenvironment, including immune cell infiltration and tumor heterogeneity.
Table 1. Comparison of immunoassay-based biomarker analysis platforms
ELISA | MSD | Luminex | Flow cytometry (CBA) | ELISpot | Simoa | GyroLab | |
Principle | Enzyme-Linked Immunosorbent Assay | Electrochemiluminescence (ECL) | Fluorescent-coded microbeads (xMAP technology) | Flow Cytometry Microbeads | Enzyme-Linked Immunospot (Cell Division) | Single Molecule Immunoassay | Microfluidic chip, automated immunoassay |
Sensitivity | pg/mL | fg/mL (highest) | pg/mL | pg/mL | Single cell level (direct count) | Ultra-high (fg/mL to ag/mL) | pg/mL |
Throughput | Standard single factor | Single well 1-10 factors | Single well 50-100 factors | Each tube 4-10 factors (some 30+) | Single well 1-2 factors | High (single test 1-6 factors, supports multiplexing) | Medium-High (5 indicators/chip, supports multi-chip parallel analysis) |
Sample Volume | 50-100 µL | 25-50 µL | 25-50 µL | 50-100 µL | 1-5 x 10^5 cells/well | 50-100 µL | 1-5 µL |
Turnaround Time | 4-6 hours | 2-4 hours | 4-6 hours | 4-6 hours | 2-3 days (including cell stimulation) | 2-3 hours | 1.5 hours |
Instrument | Microplate reader | Requires MSD dedicated instrument | Requires Luminex instrument | Standard flow cytometry instrument | Microplate reader/microscope | HD-1 analyzer | Gyrolab® xPlore |
Cost | Low (reagents) | High (instrument + reagents) | High (instrument + reagents) | Medium (reagents + instrument maintenance) | Medium (reagents + materials) | Extreme (instrument + reagents) | High (instrument + reagents) |
Advantage | Low cost, various commercial kits available | Ultra-high sensitivity, broad dynamic range | High throughput, flexible | High throughput, flexible | Cellular functionality assessment | flexible, high sensitivity, capable of detecting extremely low abundance molecules | Extremely low sample consumption, fully automated, reduces manual handling |
Limitation | Limited multiplexing, sensitivity to moving parts | Cost, common range limitation | Risk of cross-reactivity | Limited throughput | Single factor, semi-quantitative | Limited throughput, suitable for small-scale studies | Requires dedicated microfluidic chip and instrument |
Application | Routine quantitative analysis (e.g., cytokines, growth factors, antibodies) | Ultra-sensitive analysis (e.g., low abundance biomarkers) | Multiplex analysis (e.g., cellular factor risk monitoring) | Immunophenotyping, cellular functionality analysis | Vaccine efficacy assessment, infectious disease immunity monitoring | Extreme low abundance biomarkers (e.g., early disease markers, rare proteins) | Small sample volume testing (e.g., mouse serum) |
Molecular Biology-Based Biomarker Analysis
Beyond the LC-MS and immunoassay platforms discussed above, certain biomarkers, such as DNA and RNA, are more effectively analyzed using molecular biology-based technologies. Nucleic acid biomarkers play a vital role in disease diagnosis and prognosis assessment. They can be derived from tissue samples as well as body fluids such as saliva, urine, and blood (liquid biopsy). Because of their accessibility and informative value, nucleic acid biomarkers have become powerful tools for early cancer screening, monitoring tumor progression, evaluating drug efficacy, and assessing drug resistance.
Currently, branched DNA (bDNA) technology, quantitative real-time PCR (qPCR), and digital PCR (dPCR) are the primary methods for long-chain nucleic acid quantification. Among these, PCR technology (especially dPCR) offers higher sensitivity compared to bDNA technology. It is suitable not only for the safety evaluation needs of gene therapy drugs but also for assessing the potential risk of drugs crossing the blood-milk, blood-placenta, and blood-brain barriers. Furthermore, the PCR platform offers high flexibility in assay design, allowing for precise analysis of the structural integrity of specific regions of DNA or mRNA through specific primer design. With the continuous advancement of omics technologies, cutting-edge platforms such as metabolomics, genomics, proteomics, and spatial multi-omics provide richer options and support for the high-throughput and high-precision analysis of biomarkers.
"Fit-for-Purpose" Biomarker Validation Strategy
Biomarker analysis is an essential tool in the drug development process. However, due to the diversity of molecular types, varying molecular sizes, and diversity of applications, there is currently no internationally unified validation system, resulting in a diverse regulatory landscape. From a global regulatory perspective, major drug regulatory agencies have significant differences in their guidelines for biomarker assay validation. For instance, the Chinese Pharmacopoeia and the clinical bioanalysis-related regulations issued by the European Medicines Agency (EMA) do not have specific guidance documents dedicated to biomarker assay validation. In contrast, the FDA's "Bioanalytical Method Validation Guidance" issued in 2013 first explicitly proposed the "fit-for-purpose" validation strategy. The 2018 revision of this guidance further emphasized that, given the multi-dimensional application scenarios of biomarkers in drug development, the scope of validation for their analytical methods must be determined based on the specific application context, implementing an adaptive validation strategy.
Although a series of white papers have been published, the validation content for biomarker assays continues to evolve with the diversity of molecular types and the continuous advancement of analytical technologies. Currently, a risk- and benefit-based tiered validation system has gradually become an industry consensus: When biomarker data pertains to critical registration content such as drug safety, efficacy evaluation, or dose determination, full method validation must be performed. For supportive data in early research stages (such as candidate compound screening, proof-of-concept, etc.), sponsors can flexibly select validation elements based on the study objectives. This stratified validation model ensures both the reliability of critical data and development efficiency[9].
In November 2019, a specialized workshop on "Best Practices for Biomarker Assay Development and Fit-for-Purpose Validation" featured in-depth discussions on validation parameters and key statistical criteria for biomarker assays. The results showed that over 60% of experts considered parameters such as precision, accuracy, parallelism, stability, and specificity as core validation parameters; additionally, 75% of the surveyed experts supported the establishment of unified minimum standard requirements for biomarker assay validation[10-12].
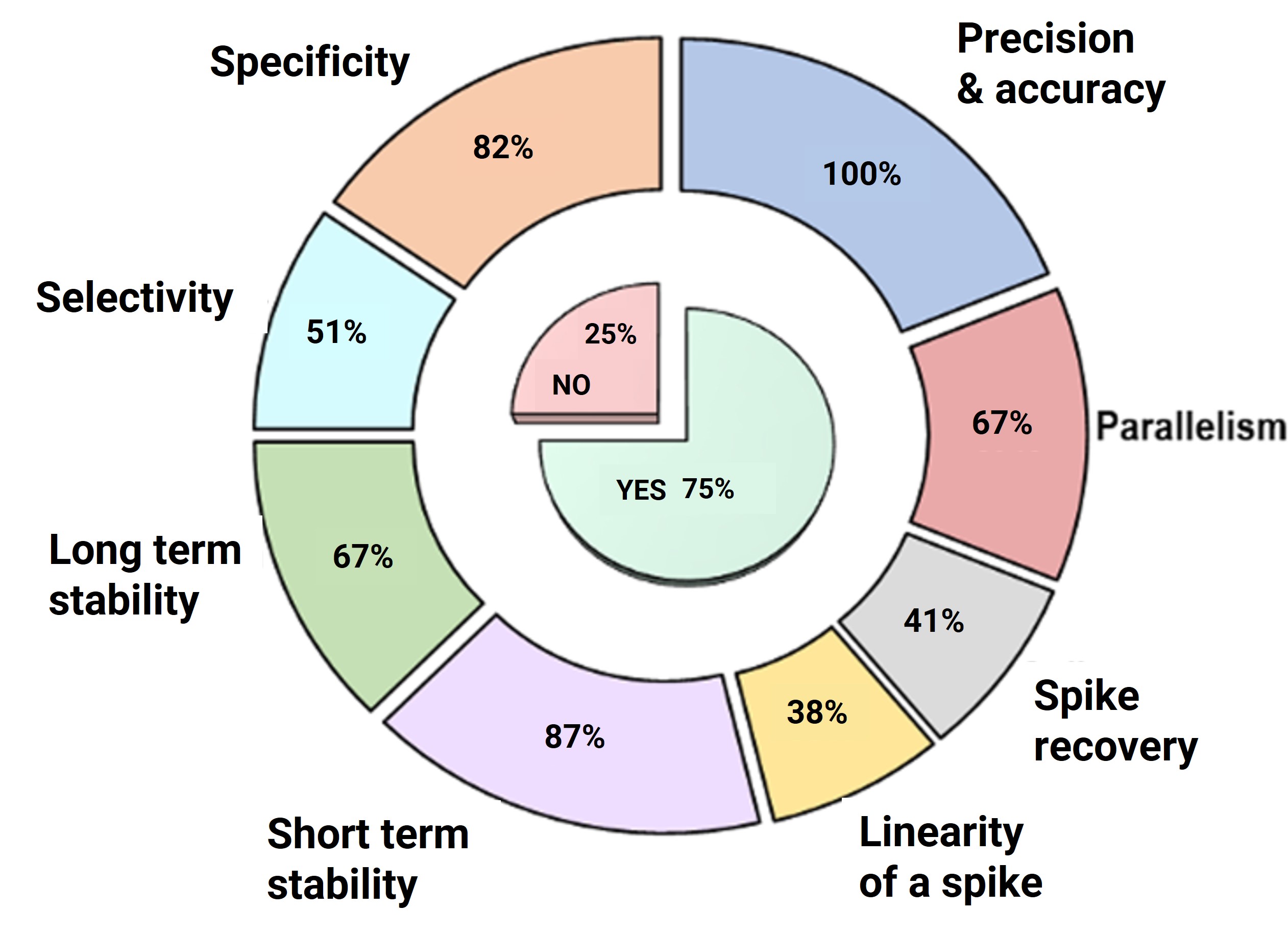
Figure 5. Workshop survey results: Key criteria for biomarker validation methods [10]
WuXi AppTec DMPK has established robust validation standards for multiple classes of biomarkers and developed tailored validation parameters according to molecular types and research purposes. For example:
Biomarker 1 (monkey serum): Validation focused on accuracy and precision, hook effect, dilutional linearity, selectivity, parallelism, and stability.
Biomarker 2 (monkey plasma): Validation parameters included accuracy and precision, hook effect, and dilutional linearity.
Biomarker 3 (monkey serum): A more comprehensive validation was performed, covering accuracy and precision, hook effect, dilutional linearity, selectivity, specificity, parallelism, robustness, and stability.
Validation Item | Validation Result | ||
Accuracy and Precision | QC concentration | Precision(%CV) | Accuracy(%Bias) |
Intra-assay Accuracy and Precision of QC (HQC/MQC/LQC) | 0.8 ~ 8.5 | 0.3 ~ 9.4 | |
Intra-assay Accuracy and Precision of QC (LLOQ and ULOQ) | 2.3 ~ 7.5 | -8.9 ~ 10.7 | |
Inter-assay Accuracy and Precision of QC ( HQC/MQC/LQC ) | 2.5 ~ 8.3 | -3.6 ~ 6.0 | |
Inter-assay Accuracy and Precision of QC (LLOQ and ULOQ) | 5.7 ~ 9.6 | -1.0 ~ 2.3 | |
Dilution Linearity | It was reliable to diluted sample in blank matrix for 5-500-fold. | ||
Hook Effect | No hook effect was observed. | ||
Selectivity (Matrix Effect) | No obvious matrix effect was observed. 10 individual lots un-spiking meet the acceptance criteria: All 10 un-spiked selectivity samples meet the acceptance criteria of %CV ≤ 20.0%. All 10 spiked selectivity samples at HQC/LLOQ concentrations meet the acceptance criteria of %Bias within ±20.0% (±25.0% for LLOQ) and %CV ≤ 20.0% (±25.0% for LLOQ). | ||
Parallelism | The %CV of back-calculated concentration of 3 diluted incurred samples met the acceptance criteria of precision ≤30.0 %. | ||
Stability | Stable for 6 hours at RT, 24 hours at 2-8 ℃,3/5 freeze/thaw cycles, and 8 days. | ||
Figure 6. Validation of experimental data for Biomarker 1 as an example
These cases illustrate that biomarker method validation is not bound to a rigid template. Instead, the validation strategy is flexibly tailored to the project’s objectives, the molecular characteristics of the biomarker, and the requirements of the development stage. This approach ensures an optimal balance between scientific rigor and practical applicability.
Closing Remarks
The biomarker testing and analysis occupy an irreplaceable role in new drug development. With continuous technological advances, analytical methods have become increasingly diverse, each offering distinct advantages and limitations. At present, WuXi AppTec has established a portfolio of biomarker analysis services spanning multiple research areas, including cytokines, neurology-related markers, hormones, oncology markers, complement system markers, and other biomarker types. This portfolio supports candidate molecules across today’s most active therapeutic fields and is backed by extensive expertise in development and testing. To date, the platform has completed quantitative analysis for hundreds of biomarkers, involving various experimental animal species such as mice, rats, rabbits, dogs, and monkeys. Key indicators are shown in the table below:
Table 2. Examples of biomarker detection capabilities across multiple species on the WuXi AppTec DMPK platform
Neurobiology & Neurodegeneration | Inflammation | Metabolic & cardiovascular | Immunology | Oncology & Cancer | Other disease related | ||
α-Synuclein Aβ40 Aβ42 Acetyl-α-Tubulin BDNF Clusterin(CLU) Neurofilament L(NFL) sAPPα sAPPβ SPTLC1 …… | CRP CXCL9 Eotaxin/CCL11 Eotaxin-3 Glutathione GM-CSF Human VEGFR1/Flt-1 iFabP IFN α IFN β IFN-γ | IL-10 IL-12/IL-23p40 IL-12p70 IL-13 IL-15 IL-16 IL-17A IL-17A/F IL-1RA IL-1α IL-1β IL-2 IL-23 | IL-4 IL-5 IL-6 IL-7 IL-8 IP-10 MCP-1 MCP-4 MDC MIP-1α MIP-1β TARC/CCL17 …… | GLP-1 Adiponectin ANGPTL3 ApoE Cortisol FBG FXI Insulin PCSK9 Total AGT Total Cholesterol Total Factor VIII Triglyceride VWF …… | Albumin C3 C3a C5 C5a CBb CFHR5 IgA IgG IgM IgE IgM TCC/C5b-9 …… | Free PSA Neurotensin NOV/CCN3 Osteopontin(OPN) Pepsinogen A Progranulin TNF α TNF-β total PSA VEGF-A TGF-β2 TGF-β3 TGF-β1 ……
| Cystatin C Growth Hormone hCG Inulin INHB IGF-I KIM-1 LH NAG NGAL Octreotide sGAG Testosterone TPO TBA …… |
Authors: Xue Zhang, Wenrui Ren, Miaomiao Song, Duo Zhang, Lili Xing
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] BEST (Biomarkers, EndpointS, and other Tools) Resource. 2016: Silver Spring (MD)Bethesda (MD).
[2] Gromova, M., et al., Biomarkers: Opportunities and Challenges for Drug Development in the Current Regulatory Landscape. Biomark Insights, 2020. 15: p. 1177271920974652.
[3] Kraus, V.B., Biomarkers as drug development tools: discovery, validation, qualification and use. Nat Rev Rheumatol, 2018. 14(6): p. 354-362.
[4] Nimse, S.B., et al., Biomarker detection technologies and future directions. Analyst, 2016. 141(3): p. 740-55.
[5] Robinson, M.R., R.A. Miller, and D.S. Spellman, Mass Spectrometry-Based Biomarkers in Drug Development. Adv Exp Med Biol, 2019. 1140: p. 435-449.
[6] Alanazi, S., Recent Advances in Liquid Chromatography-Mass Spectrometry (LC-MS) Applications in Biological and Applied Sciences. Anal Sci Adv, 2025. 6(1): p. e70024.
[7] Lasseter, H.C., et al., Cross-platform comparison of highly sensitive immunoassay technologies for cytokine markers: Platform performance in post-traumatic stress disorder and Parkinson’s disease. Cytokine: X, 2020. 2(2): p. 100027.
[8] Richens, J.L., et al., Quantitative Validation and Comparison of Multiplex Cytokine Kits. SLAS Discovery, 2010. 15(5): p. 562-568.
[9] Cummings, J., et al., Fit-for-purpose biomarker method validation for application in clinical trials of anticancer drugs. Br J Cancer, 2010. 103(9): p. 1313-7.
[10] Mathews, J., et al., Best practices for the development and fit-for-purpose validation of biomarker methods: a conference report. AAPS Open, 2022. 8(1): p. 2.
[11] Yee, L.M., T.G. Lively, and L.M. McShane, Biomarkers in early-phase trials: fundamental issues. Bioanalysis, 2018. 10(12): p. 933-944.
[12] Valentin, M.-A., et al., Validation of immunoassay for protein biomarkers: Bioanalytical study plan implementation to support pre-clinical and clinical studies. Journal of Pharmaceutical and Biomedical Analysis, 2011. 55(5): p. 869-877.
Stay Connected
Keep up with the latest news and insights.