Although peptide drugs account for only about 2% of FDA-approved therapeutics, they offer unique advantages in treating diseases where small-molecule drugs or therapeutic antibodies fall short. However, peptides are susceptible to proteolytic degradation, resulting in short half-lives. To address this, peptide chemists have introduced non-natural amino acids and chemical modifications to enhance metabolic stability. Through optimization, scientists developed GLP-1 analogs with significantly extended half-lives. Semaglutide, for example, has a half-life of 165 hours, enabling once-weekly dosing. This improvement stems not only from amino acid modifications and structural design but also from lipid conjugation, which enhances binding to plasma proteins (e.g., albumin), reducing renal clearance and prolonging systemic exposure. Thus, understanding how peptide drugs are metabolized by tissue enzymes is crucial for designing more stable therapeutics.
This article reviews in vitro metabolic stability studies of peptides in the gastrointestinal tract, liver, kidneys, and circulatory system. By analyzing commercial peptide drugs with diverse structures and molecular weights, we compare metabolic differences across tissues to guide peptide drug development.
Challenges in Peptide Drug Development
Drug metabolism significantly impacts pharmacology and safety. While peptide and small-molecule drugs follow similar pharmacokinetic and metabolic principles, key differences exist. Low-molecular-weight compounds are primarily metabolized by cytochrome P450 (CYP450) enzymes into inactive or active metabolites, whereas most peptides are degraded by proteases into amino acids [2,3]. This leads to challenges such as poor metabolic stability, high molecular weight, and limited membrane permeability, restricting their broader application [1]. Characterizing peptide-metabolizing enzymes is therefore essential. Proteolysis occurs extensively in vivo, particularly in the gastrointestinal tract, necessitating studies on enzymes that degrade peptides in the GI tract, liver, kidneys, and plasma.
In Vitro Peptide Metabolic Stability Studies Across Tissues
We selected five commercial peptide drugs with varying molecular weights, including linear and cyclic peptides (Table 1).
Table 1. The information on five commercial peptide drugs
Name | MW | FW | Type |
Somatostatin Acetate | 1637.88 | 1637.88 | Cyclic peptide |
Deslorelin Acetate | 1282.47 | 1282.47 | Linear peptide |
Octreotide Acetate | 1019.24 | 1019.24 | Cyclic peptide |
Semaglutide | 4113.64 | 4113.64 | Linear peptide (lipid-conjugated) |
Liraglutide | 3751.20 | 3751.20 | Linear peptide (lipid-conjugated) |
Peptide Metabolic Stability Studies in Plasma
Plasma is the preferred matrix for in vitro metabolic stability studies due to its accessibility. During plasma preparation, anticoagulants are added to prevent clotting. However, EDTA-K2 may inhibit certain enzyme activities, and fresh vs. frozen plasma exhibits variability. We compared three peptide drugs (fast-, medium-, and slow-metabolizing) under the following conditions:
Heparin sodium vs. EDTA-K2 anticoagulants
Fresh vs. frozen plasma
Plasma vs. serum
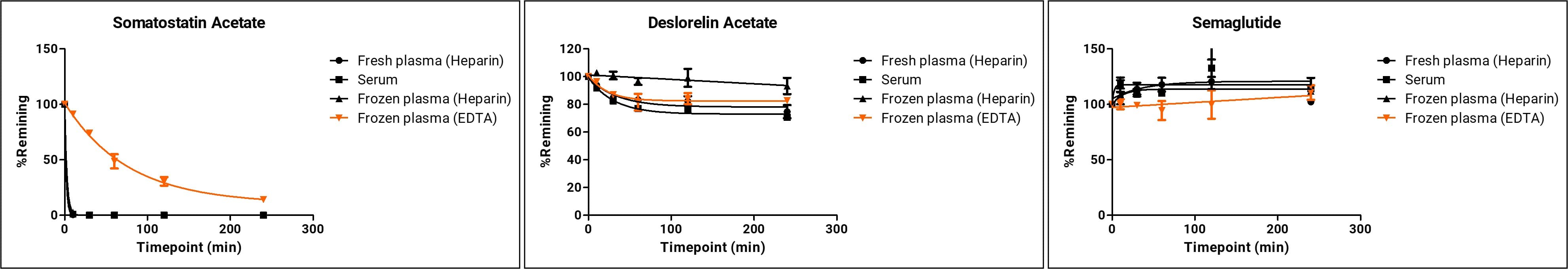
Figure 1. Metabolic stability of three peptides under different plasma conditions
Key findings: medium- and slow-metabolizing peptides showed little change across conditions, while the fast-metabolizing peptide (somatostatin) was degraded significantly more slowly in EDTA-K2-anticoagulated plasma than in sodium-heparin plasma. Based on these data, frozen plasma collected with sodium heparin is recommended for peptide metabolic stability studies. For whole-blood studies, anticoagulant choice should match the plasma experiment; sodium heparin is preferred.
Peptide Metabolic Stability Studies in Gastrointestinal (GI)
Most peptide drugs are administered via IV or subcutaneous injection due to rapid GI protease degradation and poor permeability. However, Novo Nordisk’s oral semaglutide (FDA-approved in 2019) combines with SNAC (N-[8-(2-hydroxybenzoyl)amino]caprylate), an absorption enhancer, to enable gastric absorption for peptides.
Major GI proteases relevant to peptide degradation include pepsin, trypsin, pancreatin, chymotrypsin, and elastase. WuXi AppTec DMPK offers simulated gastrointestinal fluid systems as well as single-enzyme assays to evaluate peptide stability in vitro.
We tested 12 commercial peptides against five GI enzyme systems (pepsin, trypsin, pancreatin, chymotrypsin, elastase). Table 2 summarizes half-life (t1/2) data for these peptides; the results for cyclosporine and nafarelin match literature reports, supporting assay reliability.
Table 2. Half-lives of commercial peptides in GI enzymes
Compound ID | T1/2 (min) | Literature description[4] | ||||
Chymotrypsin | Trypsin | Pancreatin | Pepsin | Elastase | ||
Cetrorelix | 390.7 | >578.1 | 62.1 | >578.1 | >578.1 | / |
Cyclosporine | >578.1 | >578.1 | >578.1 | >578.1 | >578.1 | As a cyclic peptide, cyclosporine is highly stable towards gastrointestinal peptidases (> 90% intact after 2 h incubation) |
Daptomycin | >578.1 | >578.1 | >578.1 | >578.1 | >578.1 | / |
Degarelix | >578.1 | >578.1 | >578.1 | >578.1 | >578.1 | / |
Eptifbatide | >578.1 | >578.1 | >578.1 | >578.1 | >578.1 | / |
Icatibant | 33.9 | 159.0 | 240.7 | >578.1 | >578.1 | / |
Nafarelin | 2.1 | 5.8 | 1.4 | >578.1 | 10.0 | In vitro experiments revealed it to be surprisingly stable towards human gastric peptidases (~ 90% intact after 2 h incubation) but is rapidly hydrolysed by intestinal peptidases (0% intact within 5 min) |
Somatostatin Acetate | <5.0 | 4.2 | <5.0 | >578.1 | 1.8 | / |
Deslorelin Acetate | 12.5 | 6.0 | 1.9 | >578.1 | >578.1 | / |
Semaglutide | <5.0 | 9.3 | 7.9 | 60.1 | 2.4 | / |
Liraglutide | <5.0 | 2.5 | 7.4 | >578.1 | <7.5 | / |
Peptide Metabolic Stability Studies in the Liver
The liver’s rich metabolic enzymes make it critical for drug metabolism. Common in vitro hepatic systems and their strengths/limitations are summarized below.
Table 3. Comparison of liver metabolism systems for peptide drugs
System | Pros | Cons | Recommendation for Peptides |
Liver microsomes | High CYP450 activity | Lacks cellular structure | × |
Liver S9 | Diverse proteases/hydrolases | No intracellular environment | ✓ |
Hepatocytes | Full metabolic functionality | Poor for low-permeability peptides | ✓ |
Liver homogenate | A comprehensive set of metabolic enzymes | May include non-specific components that increase background noise | ✓ |
The five peptides showed higher or comparable metabolic clearance in human and rat liver S9 fractions than in other hepatic systems. Liver S9 half-life values correlated best with in vivo results. Juha Jyrkäs et al. (2021)[5]compared four commercial peptides (cyclosporine A, leuprorelin, desmopressin, cetrorelix) across liver S9, hepatocytes, and Upcyte hepatocytes (expanded primary hepatocyte lines) and found that liver S9 produced the highest clearance and the most metabolites, supporting liver S9 as a preferred system for peptide metabolic stability studies.
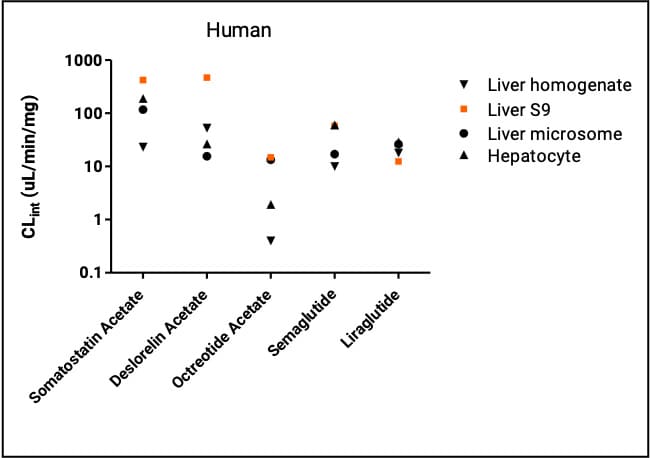
Figure 2. Clearance rates of five peptides in human liver systems
Table 4. In vitro vs. in vivo half-life correlation of somatostatin acetate and octreotide acetate
Compound ID | T1/2 (min) | Literature Description [4] | ||
Liver S9 | Hepatocyte | Liver homogenate | ||
Somatostatin Acetate | 1.6 | 7.3 | <30 | 2 to 3 min for somatostatin |
Octreotide Acetate | 46.9 | >720.0 | >3468 | When administered intravenously or subcutaneously, octreotide has a plasma half-life of 1.7 to 1.9 h, Octreotide is primarily metabolized in the liver. |
Peptide Metabolic Stability Studies in the Kidney
In vitro kidney systems and peptide clearance in vitro renal metabolism are commonly assessed using kidney microsomes, kidney S9, and kidney homogenate. Because microsomal enzyme composition is most relevant to small molecules, we focused on kidney S9 and kidney homogenate comparisons for peptides. As in the liver, the five peptides generally showed higher clearance in kidney S9 than in kidney homogenate. Juha Jyrkäs et al. (2023)[6]compared cyclosporine A, leuprorelin, and cetrorelix in kidney and intestinal matrices and found that the subcellular S9 fraction produced the fastest metabolic clearance. These findings indicate kidney S9 is a suitable system for studying peptide metabolism in extrahepatic tissues.
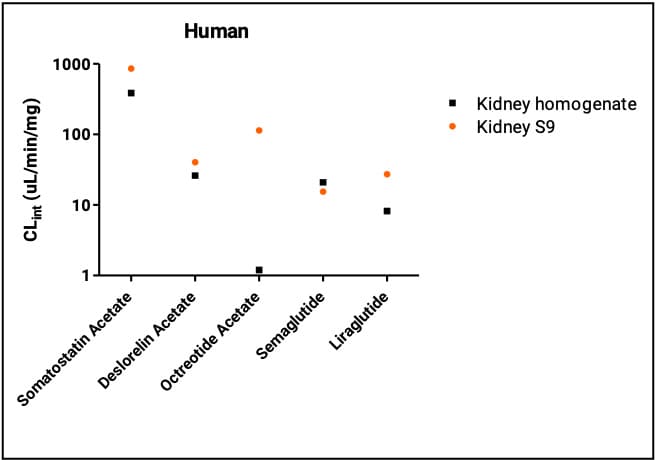
Figure 3. Clearance rates of five peptides in the human kidney system
Key findings: select the in vitro platform according to the intended route of administration and relevant tissues, then use metabolic data to design peptide modifications that improve stability—for example, N- or C-terminal capping, substitution with non-natural amino acids, cyclization, and lipidation.
Case Studies: Metabolic Stability of Approved Peptides
A review titled “A Review on the Metabolism of 25 Peptide Drugs” reported that among 25 FDA-approved peptides, 20 contain at least one non-natural amino acid to extend systemic half-life. Of those 20 peptides, 19 include D-amino-acid substitutions, and 16 have N- or C-terminal modifications to prevent exopeptidase cleavage[4]. However, exceptions exist: for instance, icatibant contains five non-natural amino acids yet still has a relatively short half-life.
Icatibant is a bradykinin B2 receptor antagonist used to treat acute attacks of hereditary angioedema (HAE). It is primarily metabolized by peptidases. Despite the inclusion of five non-natural residues, subcutaneous icatibant has an elimination half-life of approximately 1–1.8 hours (Hide et al., 2020). It is extensively proteolyzed in plasma and liver to two inactive metabolites that are excreted renally. Figure 4 shows icatibant’s structure with non-natural residues and reported endogenous protease cleavage sites.
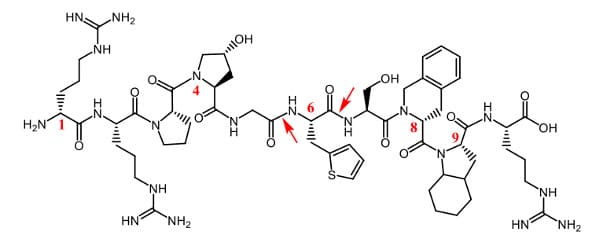
Figure 4. Icatibant structure
Red: Non-natural amino acids; arrows: reported cleavage sites.
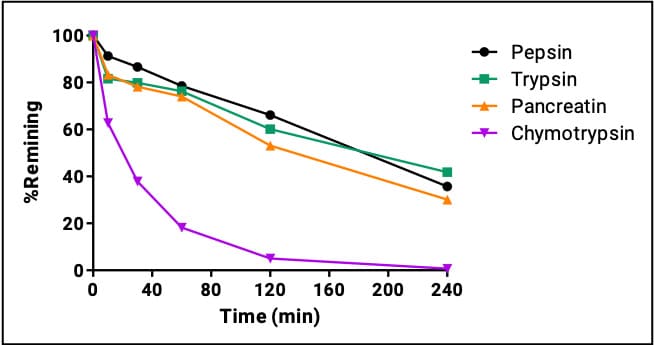
Figure 5. Icatibant’s GI enzyme stability
Daptomycin is a cyclic lipopeptide antibiotic composed of a 13-residue cyclic peptide (including seven non-natural amino acids) and a decanoyl side chain. It is indicated for complicated skin and skin-structure infections (cSSSI), Staphylococcus aureus bloodstream infections (including right-sided infective endocarditis), and other severe infections by resistant Gram-positive bacteria. Daptomycin’s plasma half-life is about 6.6–8.8 hours, enabling once-daily intravenous dosing (2011 labeling). Its longer half-life is largely due to high plasma protein binding (90-95%), which limits glomerular filtration. Daptomycin’s resistance to GI proteases is shown in Figure 7, consistent with the stabilizing effect of non-natural amino acids.
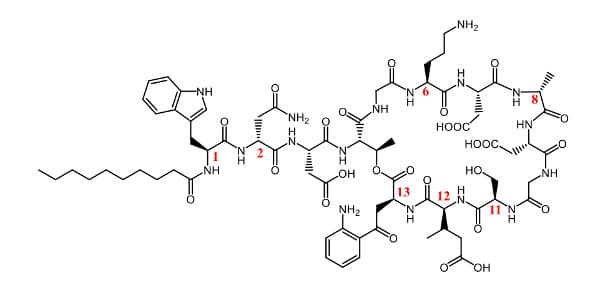
Figure 6. Daptomycin structure
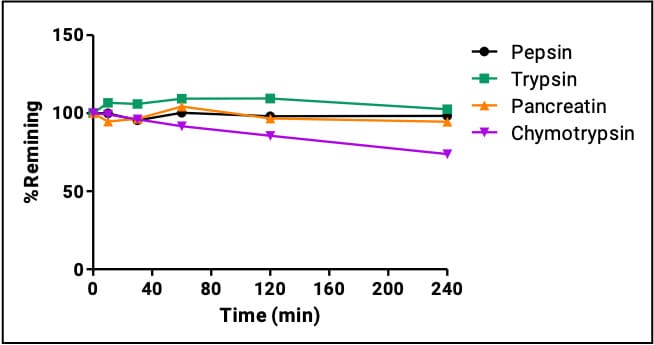
Figure 7. Daptomycin’s GI enzyme stability
Summary
Compared with small molecules, peptide drugs often show higher potency and selectivity and can address complex disease targets. Compared with protein biologics, peptides generally offer better manufacturability, higher purity, and lower immunogenicity. WuXi AppTec DMPK provides peptide-focused strategies for screening, lead optimization (PCC), and IND stages, and has established comprehensive peptide DMPK platforms that include stability studies in circulation, gastrointestinal fluid/enzyme systems, liver, and kidney. These services enable clients to access one-stop, efficient solutions for peptide metabolism and stability assessment.
Authors: Zhengu Xu, Haijuan Liu, Xiangling Wang, Genfu Chen
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Lau JL, Dunn MK (2018) Therapeutic peptides: historical perspectives, current development trends, and future directions. Bioorg Med Chem 26(1):2700–2707
[2] M. Werle, (2006) Strategies to improve plasma half-life time of peptide and protein drugs. Amino Acids. 30: 351–367
[3] Jin-Feng Yao, Hong Yang, (2018) Metabolism of Peptide Drugs and Strategies to Improve their Metabolic Stability. Current Drug Metabolism. 19: 892-901
[4] C. S. Brian Chia (2021), A Review on the Metabolism of 25 Peptide Drugs. International Journal of Peptide Research and Therapeutics. 27:1397–1418
[5] Juha Jyrkäs, (2021) Hepatic in vitro metabolism of peptides; Comparison of human liver
S9, hepatocytes, and Upcyte hepatocytes with cyclosporine A, leuprorelin, desmopressin, and cetrorelix as model compounds. Journal of Pharmaceutical and Biomedical Analysis. 196: 113921
[6] Juha Jyrkäs, (2023) Extrahepatic in vitro metabolism of peptides; comparison of human kidney
and intestinal S9 fraction, human plasma and proximal tubule cells, using cyclosporine A, leuprorelin, and cetrorelix as model compounds. Journal of Pharmaceutical and Biomedical Analysis. 225: 115219
Related Services and Platforms




-

 In Vitro ADME ServicesLearn More
In Vitro ADME ServicesLearn More -

 Novel Drug Modalities DMPK Enabling PlatformsLearn More
Novel Drug Modalities DMPK Enabling PlatformsLearn More -

 Physicochemical Property StudyLearn More
Physicochemical Property StudyLearn More -

 Permeability and Transporter StudyLearn More
Permeability and Transporter StudyLearn More -

 Drug Distribution and Protein Binding StudiesLearn More
Drug Distribution and Protein Binding StudiesLearn More -

 Metabolic Stability StudyLearn More
Metabolic Stability StudyLearn More -

 Drug Interactions StudyLearn More
Drug Interactions StudyLearn More
Stay Connected
Keep up with the latest news and insights.











