Central nervous system (CNS) disorders affect a vast patient population. Yet, the clinical success rate for CNS drugs is only about 8%, less than half that of drugs targeting cardiovascular or infectious diseases [1]. A key reason is the poor predictive accuracy of preclinical animal models for human CNS drug exposure. Microdialysis (MD) has become a cutting-edge sampling technique for evaluating the concentration changes of endogenous and exogenous substances in the body, as well as studying metabolic, biochemical, and pharmacological events in tissues. This article introduces the principles of brain microdialysis and its application in pharmacokinetics (PK) research in NHPs and rodents.
What is Brain Microdialysis?
The brain microdialysis is used to directly obtain free drug concentrations in the brain. A microdialysis probe containing a dialysis membrane is implanted into the brain, which is continuously perfused with artificial extracellular fluid (Figure 1). Due to the concentration gradient on both sides of the dialysis membrane, the substances in the brain will be extracted by the exchange. This model allows continuous sampling in conscious animals, and the free drug concentrations in the brain can be monitored in a real-time manner. In addition, if dialysis of the brain and blood is performed simultaneously on the same animal, Kp, uu could be obtained directly.
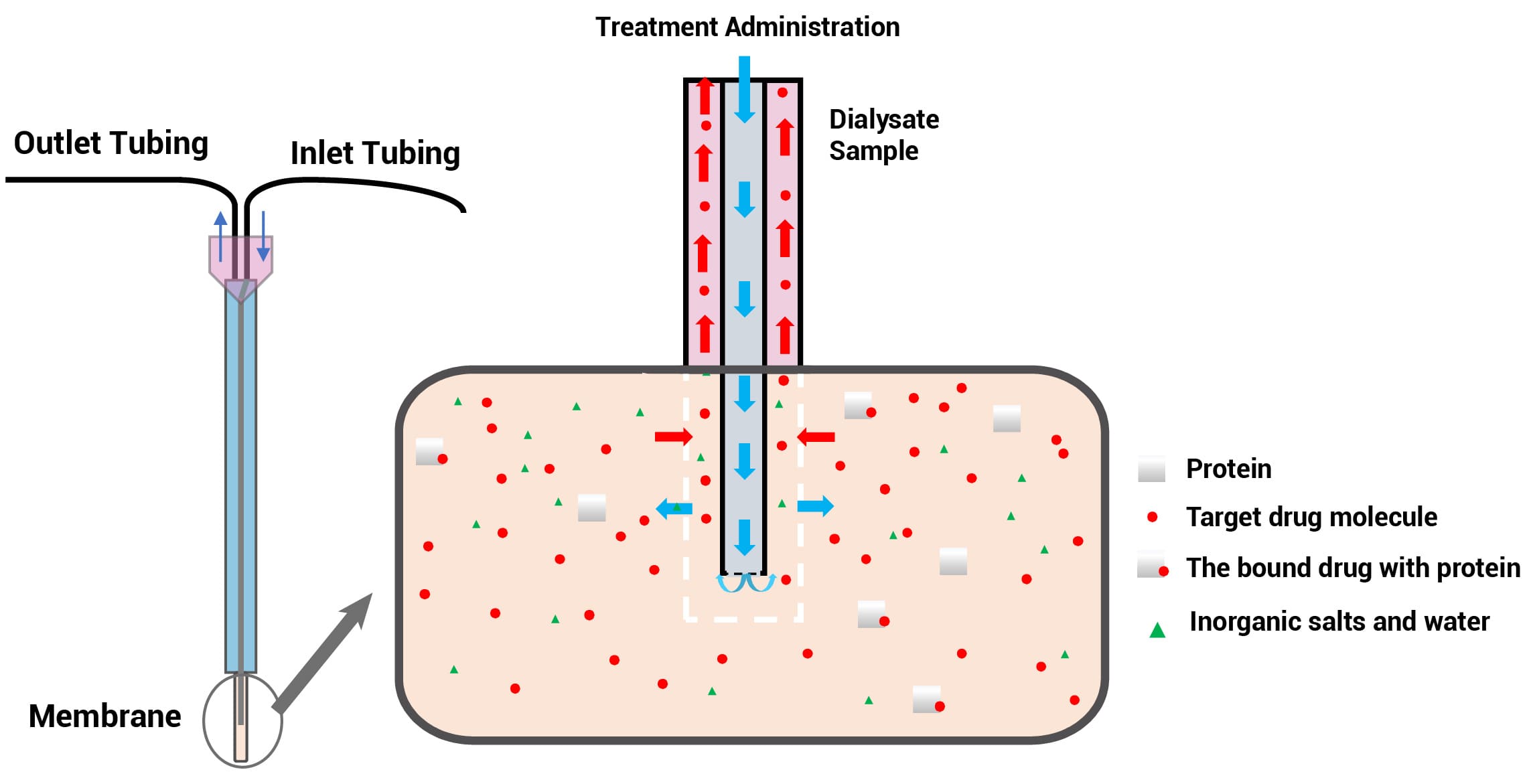
Figure 1. Schematic of the brain microdialysis principle
Brain Microdialysis Device
The microdialysis system device (Figure 2) is mainly composed of a micro-pump, microdialysis probe, collector, connecting tube, and supporting equipment. The microdialysis probe is the core of this technology. Until 2024, the commercially available probes are concentric circle probes, linear probes, and ring probes, among which concentric probes are primarily used in the brain and blood; linear probes are mainly used in peripheral tissues, such as skin, eyes, and muscles. The most widely used device is the concentric circular probe, whose structure is similar to a capillary. The probe comprises an inlet tube, an outlet tube, a shaft, and a tip vessel implanted into the tissue. The vessel with a semi-permeable (white) tip is where the free drug in the interstitial space and the probe liquid are exchanged.

Figure 2. Schematic diagram of the microdialysis device and Probe
Case Study 1: Brain Microdialysis in Rodents
CSF concentrations are often used as a surrogate for free brain drug concentrations, but this approach can be inaccurate, particularly for compounds that are substrates of transporters. Microdialysis enables simultaneous monitoring of unbound drug in both brain interstitial fluid and blood in the same animal, providing valuable insight into brain penetration. The application of microdialysis in CNS drug research, which can simultaneously monitor free drug concentrations in the brain and blood from the same animal, is of great value in elucidating the permeability of the compound into the brain.
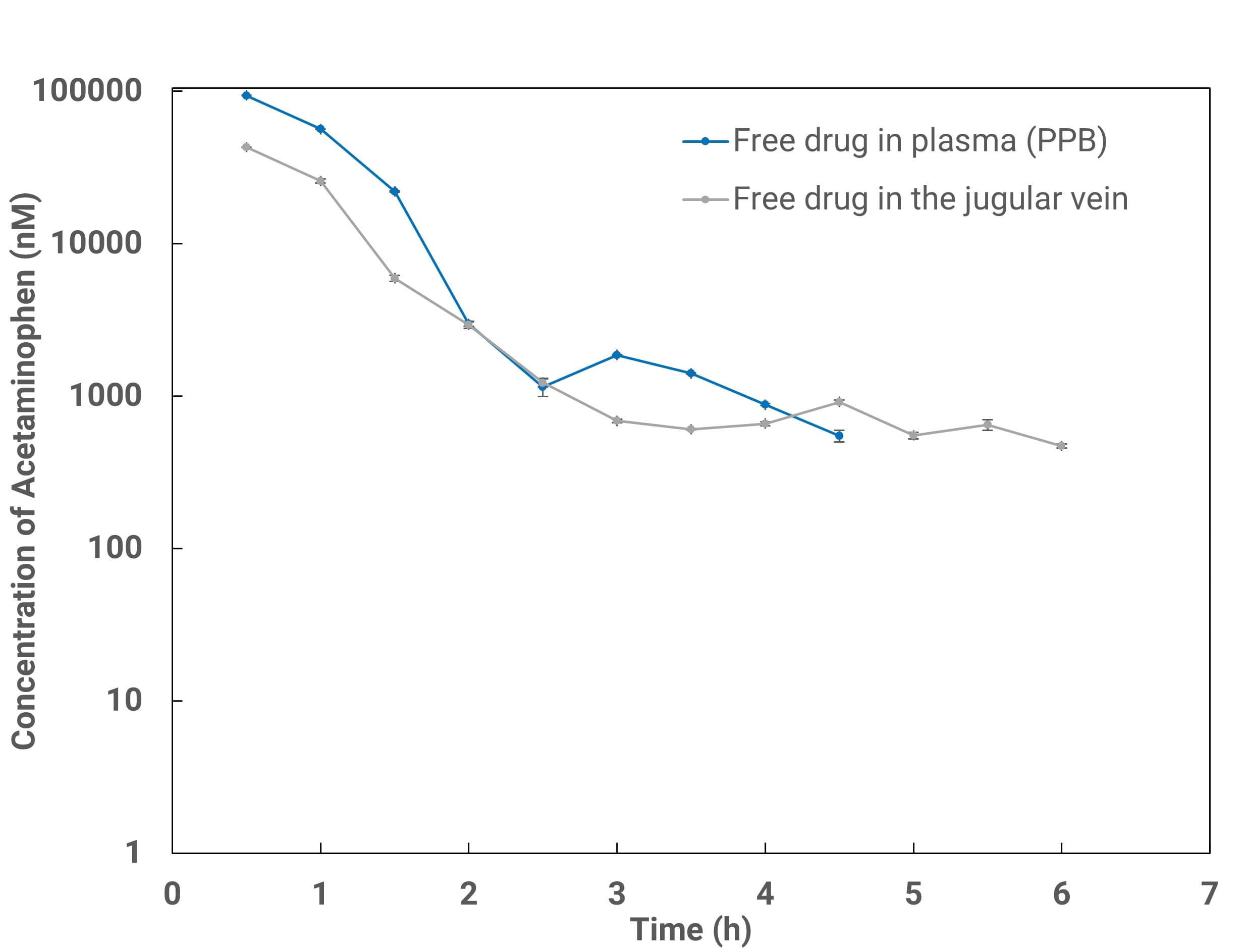
Figure 3. Comparison of free plasma concentrations measured by serial bleeding (blue; corrected by in vitro plasma protein binding fp,u) and by microdialysis probe in the jugular vein (gray).
Figure 3 shows good agreement between MD-determined free plasma concentrations and serial-bleeding results corrected for plasma fraction unbound. Combining brain MD with blood MD (via intravenous cannulation) in the same animal yields unbound concentrations in brain and plasma, unbound AUC, and Kp,uu (Figure 4). Biomarkers such as neurotransmitters can also be measured concurrently to explore PK/PD relationships.
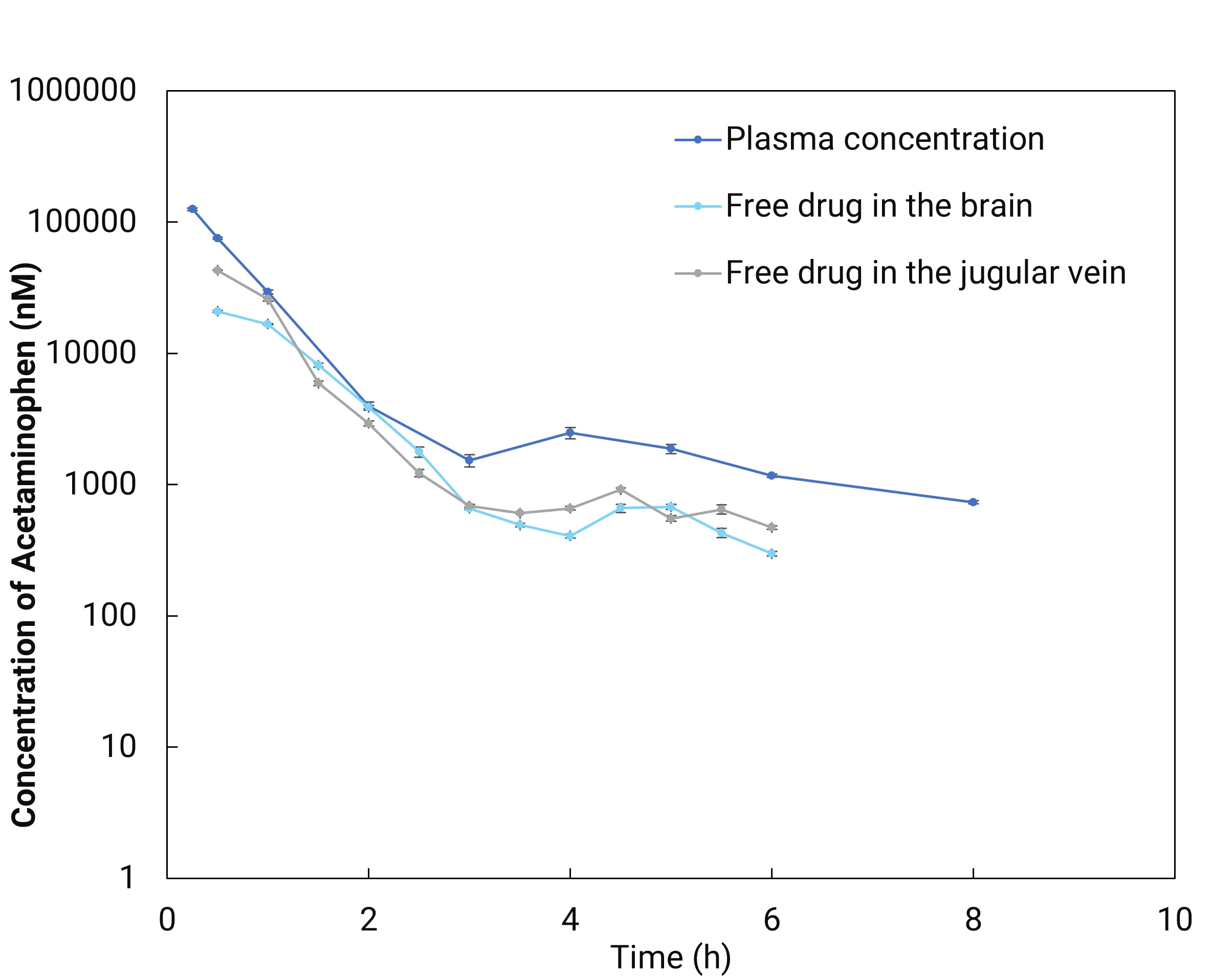
Figure 4. Brain microdialysis in combination with venous cannula (blood) microdialysis (dark blue line: total drug concentrations in plasma collected by serial bleeding; light blue line: free-drug concentrations in brain obtained directly by microdialysis probe in the brain; grey line: free-drug concentrations in blood obtained directly by microdialysis probe in the jugular vein).
Case Study 2: Brain Microdialysis in NHPs
Non-human primates (such as monkeys) have brain structures more similar to those of humans, including the cerebral cortex, limbic system, and other important regions, making their brain tissue distribution characteristics closer to humans. As shown in Table 1, the expression levels of key transport proteins P-gp and MRP4 in cynomolgus monkeys are more similar to those in humans than in rats.
Table 1. Expression levels of key transport proteins P-gp and MRP4 on the blood-brain barrier of rats, cynomolgus monkeys, and humans [2-4]
Membrane Protein | Expression Level on Blood-Brain Barrier Membrane (fmol/g protein) | ||
Rats | Cynomolgus monkeys | Humans | |
P-gp | 19.1 | 4.71 | 6.06 |
MRP4 | 1.53 | 0.286 | 0.195 |
Monkey brain microdialysis involves implanting a microdialysis probe in the target area. As shown in Figure 5, the target area at the probe tip is the striatum. The probe usually consists of a small semipermeable membrane tube that selectively allows substances smaller than the membrane pore size to pass through. After injecting artificial cerebrospinal fluid into the probe, substances in the brain will pass through the semipermeable membrane into the solution inside the probe, allowing for collection and analysis.
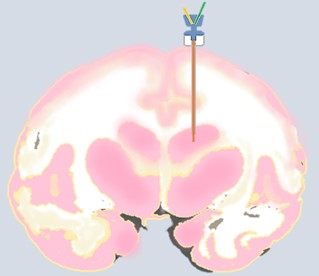
Figure 5. Schematic diagram of brain microdialysis in NHPs
Based on reported monkey brain microdialysis techniques [5], combined with existing microdialysis technology reserves and rodent microdialysis experience, carbamazepine, a non-P-gp substrate that enters the brain through passive diffusion, was selected as the validation drug. If the measured free drug concentration in the brain is comparable to the free drug concentration in plasma, it can prove the reliability of monkey brain microdialysis operations.
After administering 15 mg/kg and 7.5 mg/kg of carbamazepine to cynomolgus monkeys via nasal gavage, blood and striatum microdialysis fluid were collected to evaluate the distribution of the drug in plasma and monkey brain tissue. As shown in Figure 6, the Cmax of extracellular fluid (ECF) in the brain was 982 ng/mL and 531 ng/mL at doses of 15 mg/kg and 7.5 mg/kg, respectively, showing a clear dose-concentration gradient relationship. The Cmax was slightly higher than the literature values (693.4 ng/mL and 321.6 ng/mL at the same doses [5]). The ratio of drug exposure in ECF to free drug exposure in plasma (AUC0-5h) was 82.0% and 77.5% at doses of 15 mg/kg and 7.5 mg/kg, respectively, consistent across both doses and slightly lower than the literature value (113.9±7.5% [5]). The difference from the literature results may be related to the monkey species.
In summary, in the monkey brain microdialysis technique, the Cmax of extracellular fluid in the monkey brain and the free drug exposure AUC0-5h in the brain are basically consistent with the literature, and the PK parameters of the two different concentrations have a good dose gradient relationship. The ratio of drug concentration in the extracellular fluid of the human brain to the free drug concentration in plasma is about 100% [6], which is close to the monkey brain data measured in this experiment.
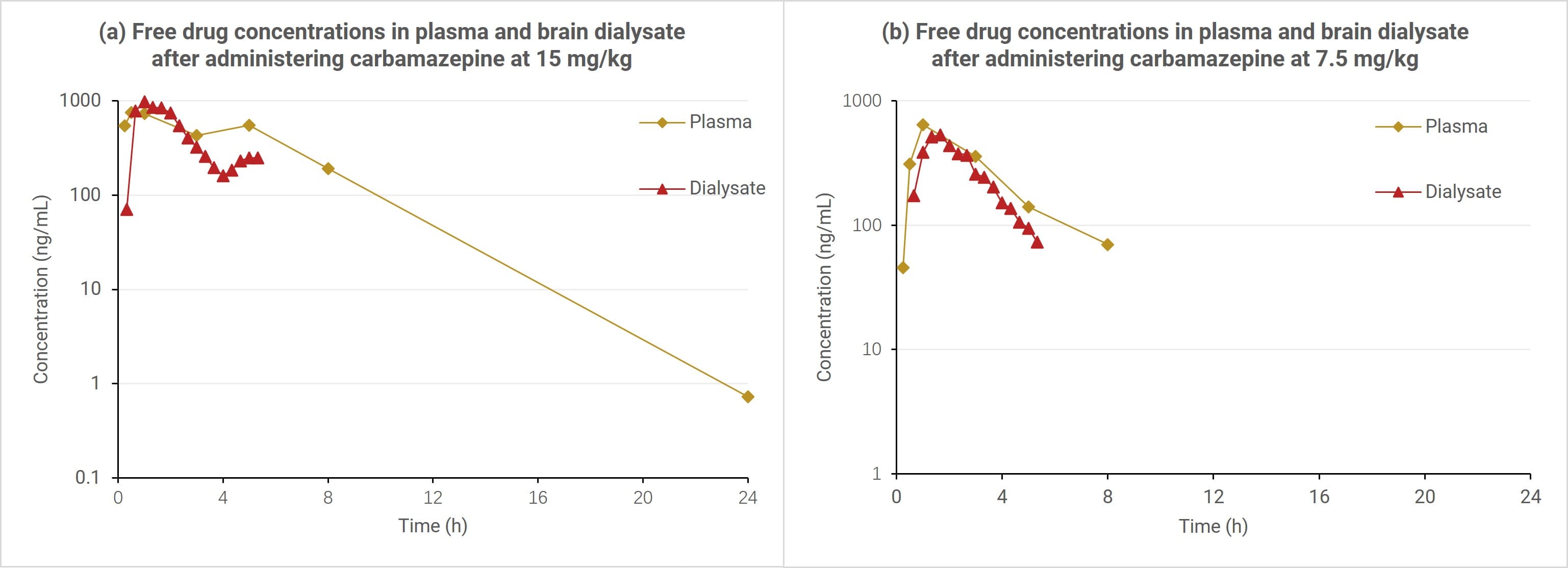
Figure 6. Time-concentration curves of carbamazepine in plasma and brain dialysis fluid in cynomolgus monkeys
A Final Word
As CNS drug discovery targets increasingly complex mechanisms, microdialysis in rodents and NHPs offers a critical tool for bridging the translational gap. By providing direct measurements in physiologically relevant models, this approach may finally help overcome the historical challenges of CNS drug development. WuXi AppTec DMPK not only has the capability of brain microdialysis but also has established a comprehensive CNS drug development platform with various CNS drug administration and sampling techniques, such as intrathecal injection, lateral ventricle administration, and cerebellomedullary cistern CSF collection, to fully support the development of new CNS drugs.
Authors: Furong Jiao, Bin Xu, Liping Tang, Xuan Dong, Qigan Cheng, Jianping Sun, Cheng Tang, Shoutao Liu, Jing Jin
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
1 Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004 Aug;3(8):711-5. doi: 10.1038/nrd1470. PMID: 15286737.
2 Ito K, Uchida Y, Ohtsuki S, Aizawa S, Kawakami H, Katsukura Y, Kamiie J, Terasaki T. Quantitative membrane protein expression at the blood-brain barrier of adult and younger cynomolgus monkeys. J Pharm Sci. 2011 Sep;100(9):3939-50. doi: 10.1002/jps.22487. Epub 2011 Jan 19. PMID: 21254069.
3 Hoshi Y, Uchida Y, Tachikawa M, Inoue T, Ohtsuki S, Terasaki T. Quantitative atlas of blood-brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J Pharm Sci. 2013 Sep;102(9):3343-55. doi: 10.1002/jps.23575. Epub 2013 May 6. PMID: 23650139.
4 Syvänen S, Lindhe O, Palner M, Kornum BR, Rahman O, Långström B, Knudsen GM, Hammarlund-Udenaes M. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab Dispos. 2009 Mar;37(3):635-43. doi: 10.1124/dmd.108.024745. Epub 2008 Dec 1. PMID: 19047468.
5 Thiollier T, Wu C, Porras G, Bezard E, Li Q, Zhang J, Contamin H. Microdialysis in awake macaque monkeys for central nervous system pharmacokinetics. Animal Model Exp Med. 2018 Dec 4;1(4):314-321. doi: 10.1002/ame2.12046. PMID: 30891581; PMCID: PMC6388052.
6 Scheyer RD, During MJ, Spencer DD, Cramer JA, Mattson RH. Measurement of carbamazepine and carbamazepine epoxide in the human brain using in vivo microdialysis. Neurology. 1994 Aug;44(8):1469-72. doi: 10.1212/wnl.44.8.1469. PMID: 8058151.
Related Services and Platforms




-

 In Vivo PharmacokineticsLearn More
In Vivo PharmacokineticsLearn More -

 Therapeutic Areas DMPK Enabling PlatformsLearn More
Therapeutic Areas DMPK Enabling PlatformsLearn More -

 Rodent PK StudyLearn More
Rodent PK StudyLearn More -

 Large Animal (Non-Rodent) PK StudyLearn More
Large Animal (Non-Rodent) PK StudyLearn More -

 Clinicopathological Testing Services for Laboratory AnimalsLearn More
Clinicopathological Testing Services for Laboratory AnimalsLearn More -

 High-Standard Animal Facilities and Animal WelfareLearn More
High-Standard Animal Facilities and Animal WelfareLearn More -

 Preclinical Formulation ScreeningLearn More
Preclinical Formulation ScreeningLearn More
Stay Connected
Keep up with the latest news and insights.











