Messenger RNA (mRNA) is a single-stranded ribonucleic acid transcribed from one strand of DNA. It carries genetic information and directs protein synthesis. In eukaryotes, a complete mRNA molecule consists of a 5' cap, 5' untranslated region (5' UTR), coding region, 3' untranslated region (3' UTR), and a poly(A) tail. As an emerging class of therapeutics, mRNA’s applications cover critical research areas such as infectious disease vaccines, mRNA cancer vaccines, gene therapy, and protein replacement therapy due to its excellent programmability and safety. In response to the COVID-19 pandemic, the FDA granted expedited approval to two mRNA vaccines: BioNTech/Pfizer’s BNT162b2 (COMIRNATY®) and Moderna’s mRNA-1273 (SPIKEVAX®). Nonclinical data supporting their development are accessible in another article: FDA Approved mRNA Vaccines: Interpretation of Preclinical Pharmacokinetic (PK) Data.
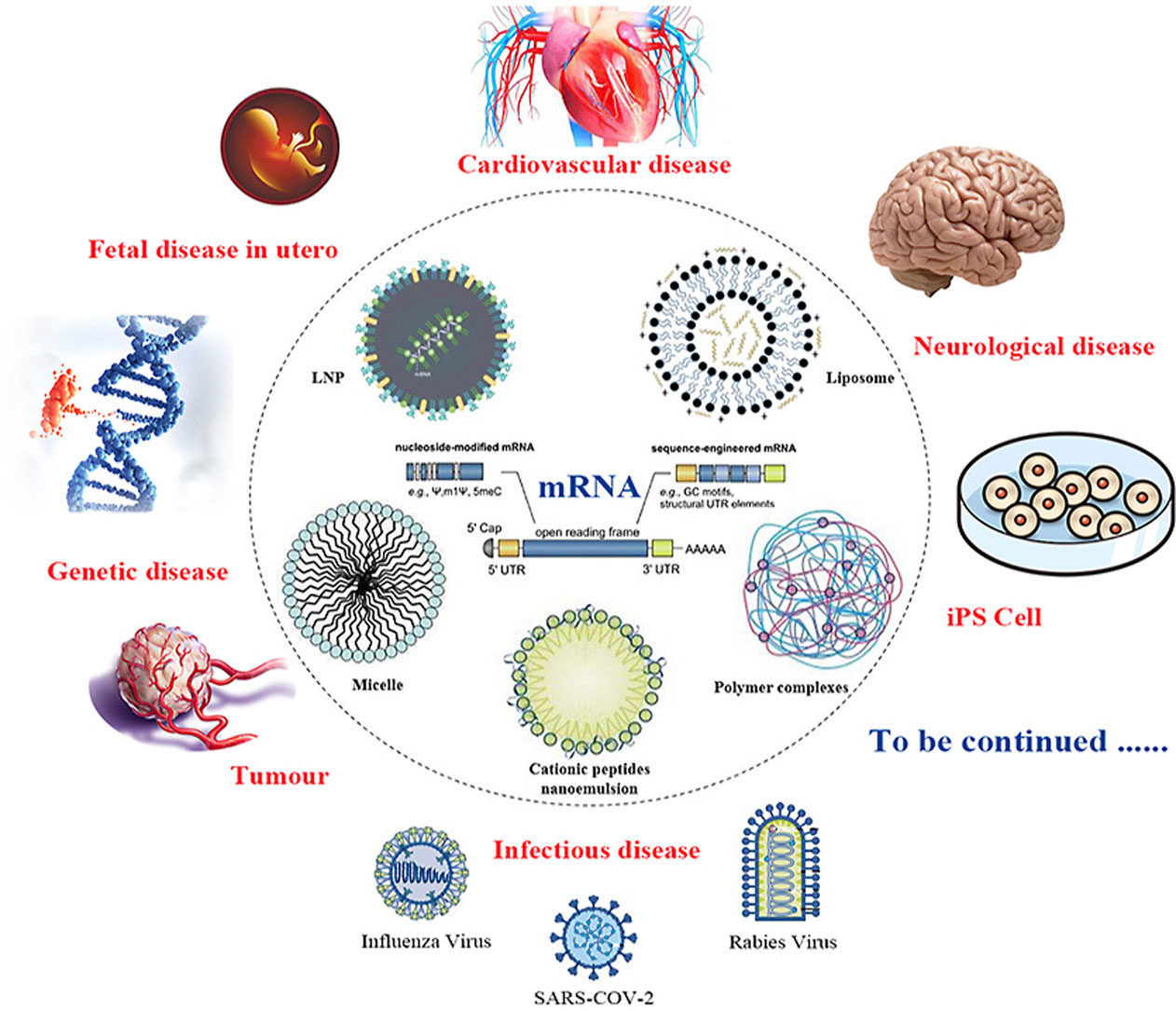
Figure 1. mRNA-targeted delivery systems and their application areas[1].
Chemical modification of mRNA structures can enhance the stability and targeting of certain drugs in vivo, improve delivery efficiency, and reduce immunogenicity. mRNA-based carriers facilitate drug delivery to disease sites, promote endosomal escape of nucleic acid therapeutics, and enhance their efficacy. This article reviews research on mRNA modification, delivery, and therapeutic applications, and elaborates on analytical methods for assessing capping efficiency, delivery systems, and quantitative analysis to advance mRNA vaccine and therapeutic development.
mRNA Modifications
mRNA therapeutics demonstrate significant potential for preventing and treating various diseases. Their stability and translational efficiency are particularly crucial. To protect mRNA from degradation by nuclease and enable tissue- or cell-specific targeting for clinical translation, structural and nucleotide modifications are essential.
Key mRNA modifications include:
1. Replacement of natural uridine with Pseudouridine (Ψ) in the 5' untranslated region (5' UTR).
2. Replacement of natural uridine/cytidine in the open reading frame (ORF) with:
N1-Methylpseudouridine (m1Ψ)
5-Methoxyuridine (mo5U)
2-Thiouridine (s2U)
5-Methylcytosine (m5C)
5-Hydroxymethylcytidine (hm5C)
3. Replacement of natural adenosine in the 3' untranslated region (3' UTR) with:
N6-Methyladenosine (m6A)
N1-Methyladenosine (m1A)
4. 5' cap modification: The 5' cap structure is vital for transcriptional initiation and mRNA stability. The discovery of novel cap analogs will significantly advance the development of mRNA vaccines and therapeutics.
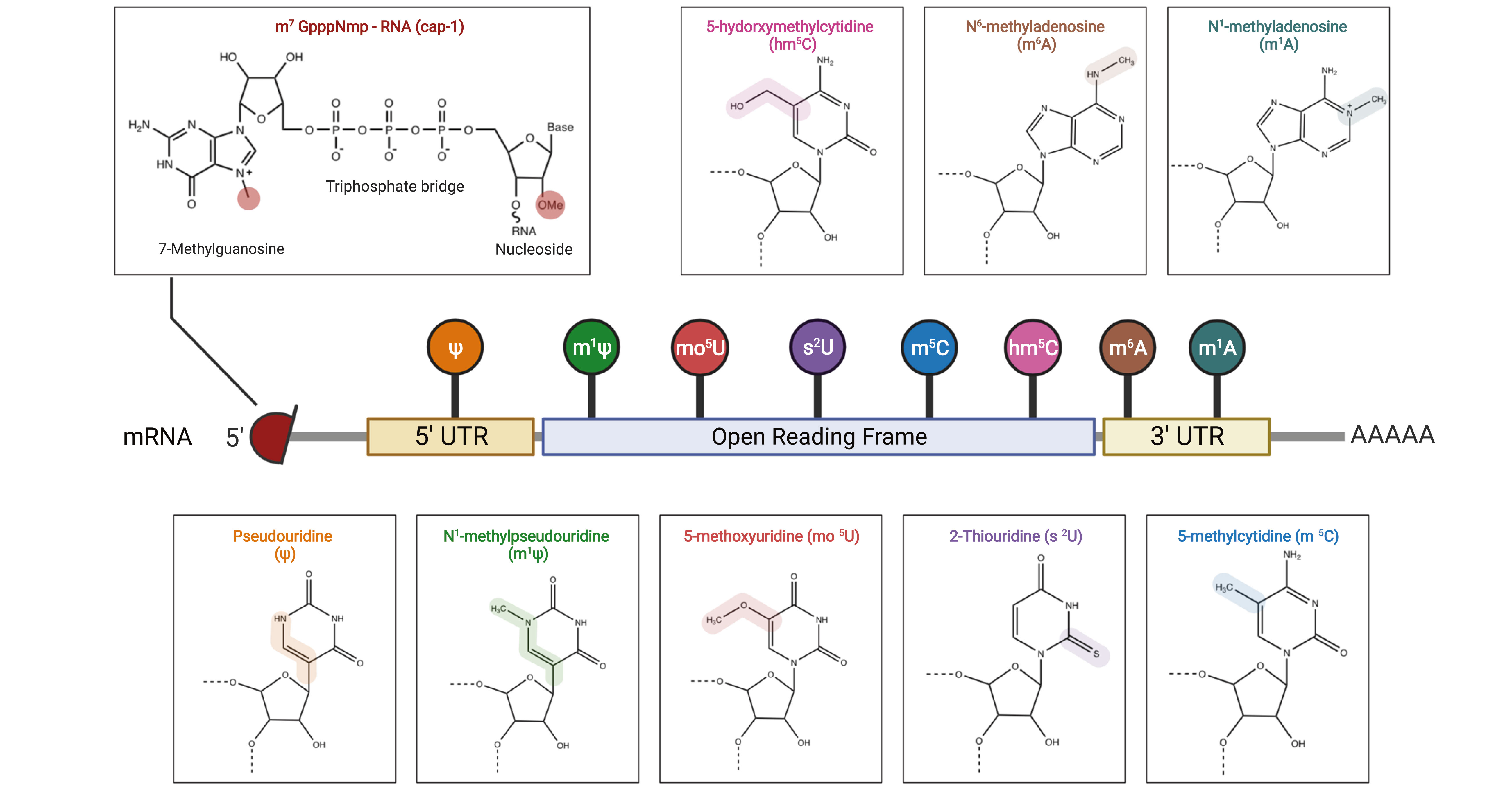
Figure 2. mRNA structure and nucleotide modifications[2].
The 5' cap (m7Gppp) at the mRNA's 5' end features a 7-methylguanosine (m7G) linked via a 5'-5' triphosphate bridge (ppp). Optimization strategies targeting the m7G moiety or triphosphate bridge enhance mRNA stability and translation efficiency. These modifications reduce immunogenicity while improving stability, intracellular expression, and delivery efficiency.
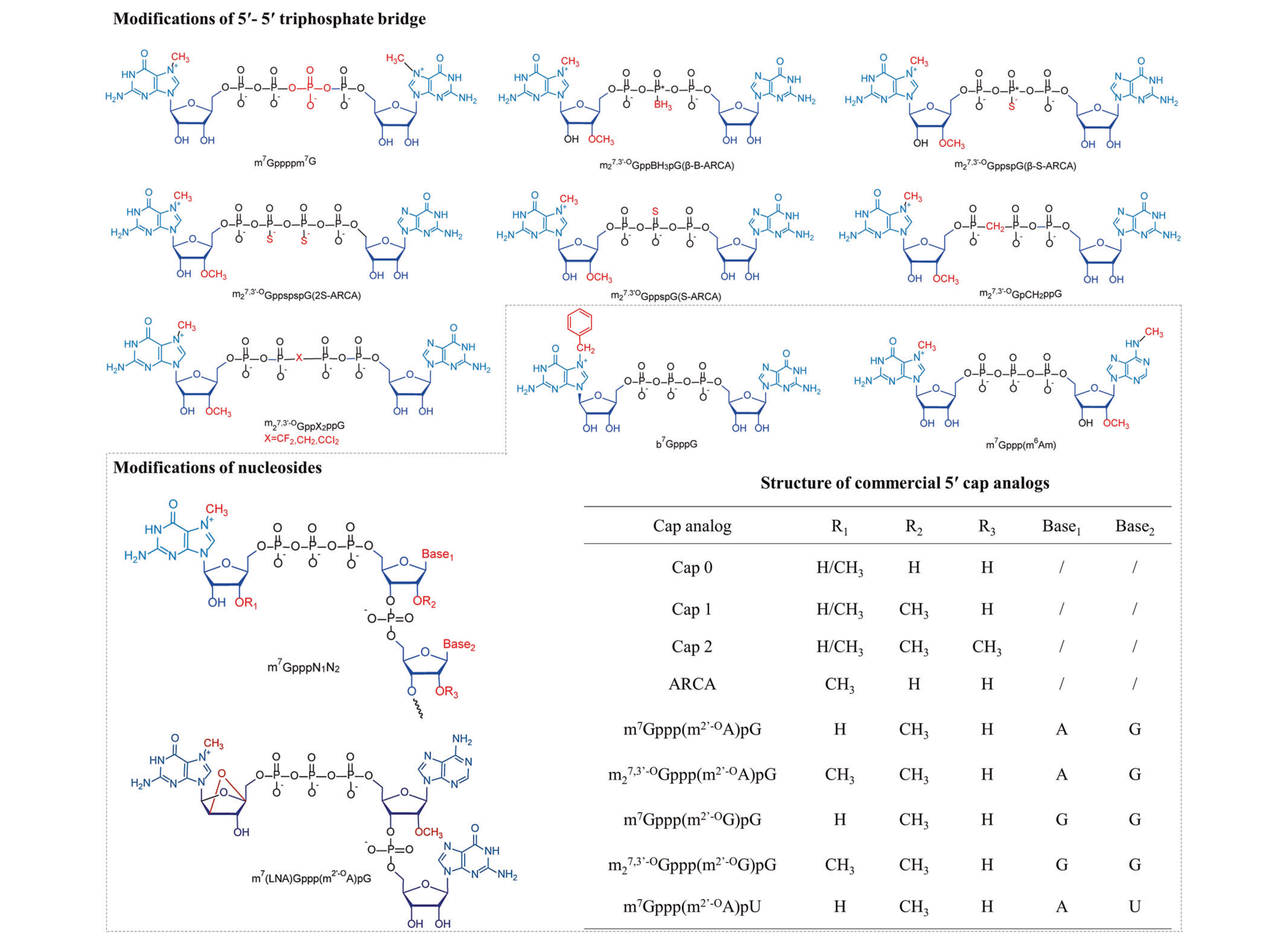
Figure 3. 5' cap structure modifications [3].
mRNA Delivery
mRNA modification and delivery systems synergistically impact therapeutic efficacy. As large, negatively charged biomolecules, mRNA cannot directly enter cells or be injected systemically without protection. Efficient delivery systems are essential to shield mRNA from nucleases and enhance cellular uptake and endosomal escape. Current delivery platforms include lipid nanoparticles (LNPs), polymer nanoparticles, inorganic nanoparticles, and protein-based virus-like nanoparticles, with LNPs being the most widely used.
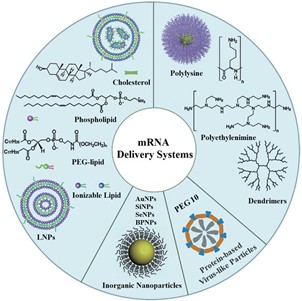
Figure 4. Schematic diagram of representative mRNA delivery systems [4].
Among these, lipid nanoparticles (LNPs) are the most prominent. A typical LNP formulation comprises:
Ionizable cationic lipids: Facilitate nucleic acid encapsulation within LNPs and mediate endosomal membrane disruption for cytosolic release. Their positive charge also promotes interaction with negatively charged cell membranes and binding to plasma proteins, aiding cellular uptake[5].
PEGylated lipids: Hydrophilic synthetic polymers that improve solubility, extend circulation half-life, reduce clearance by the reticuloendothelial system, enhance pharmacokinetics, and boost efficacy.
Helper lipids: Stabilize LNPs during storage and circulation.
Cholesterol: Mediates LNP endocytosis, maintains bilayer integrity, and ensures lipid fluidity.
State-of-the-art ionizable lipids are positively charged at low pH (facilitating mRNA complexation and stability) but neutral at physiological pH (reducing interactions with blood cell membranes and lipid toxicity). Upon cellular uptake, the acidic endosomal environment protonates these lipids, promoting mRNA release. To lower therapeutic doses and minimize toxicity, nanoparticles can be surface-functionalized with targeting ligands for specific tissue or cell receptor recognition.
Therapeutic Mechanisms of mRNA Vaccines and Therapeutics
Optimizing mRNA modification and delivery significantly enhances therapeutic efficacy and safety, positioning mRNA as a powerful modality for intractable diseases, including infectious diseases, inherited metabolic disorders, cancer, cardiovascular diseases, and others.
Driven by the success of mRNA vaccines during the COVID-19 pandemic, their future applications are expanding. The mechanism of mRNA vaccines involves:
Endocytosis of injected mRNA by antigen-presenting cells (APCs).
Cytosolic translation of mRNA into antigenic proteins by ribosomes.
Intracellular antigens are processed by the proteasome into peptides, presented on MHC class I molecules to cytotoxic T cells (CD8+ T cells).
Activated cytotoxic T cells eliminate infected cells via perforin, granzymes, etc.
Secreted antigens are endocytosed by APCs, degraded in endosomes, and presented on MHC class II molecules to helper T cells (CD4+ T cells).
Helper T cells stimulate B-cell antibody production and activate phagocytes (e.g., macrophages) via inflammatory cytokines, clearing circulating pathogens.
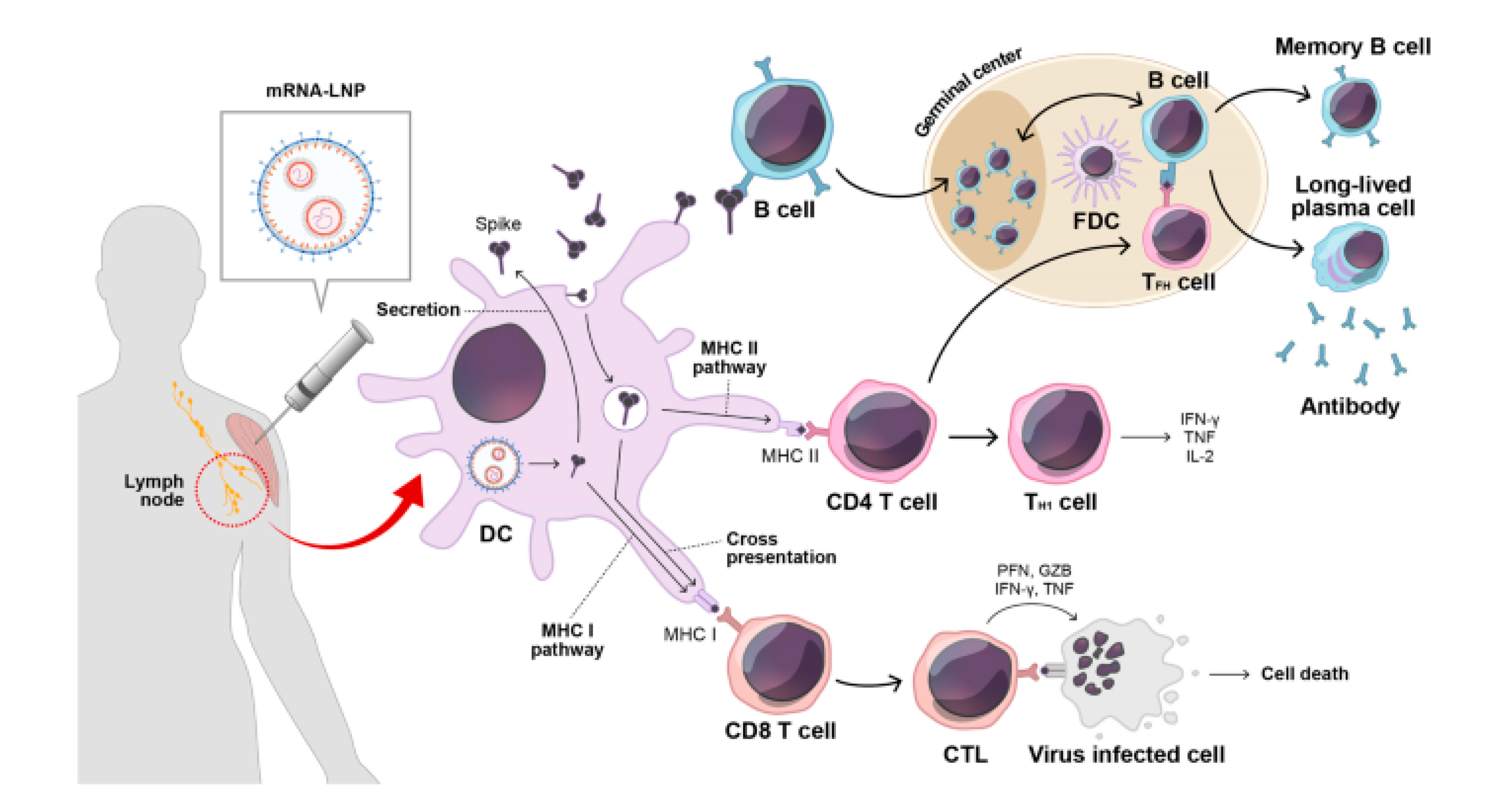
Figure 5. Mechanism of mRNA vaccines in eliciting cell-mediated and antibody-mediated immunity[6].
The success of mRNA vaccines has also facilitated the development of other therapeutics, including:
Cancer therapeutics: Prophylactic and therapeutic cancer vaccines.
Other areas: Rare diseases, cardiovascular disorders, and autoimmune diseases.
Despite its promise, challenges remain, such as mRNA stability and the efficiency/safety of delivery systems. Thus, rigorous design, delivery optimization, and quantitative analysis are critical.
Bioanalysis of mRNA Vaccines and Therapeutics
As a novel therapeutic class, mRNA vaccines and therapeutics require systematic evaluation to ensure the efficacy and safety of modifications and delivery, enabling optimized design and improved success rates. Analytical strategies focus on three key areas:
LC-MS/MS quantification of lipids in mRNA delivery systems.
LC-HRAM-based mRNA capping efficiency assay.
RT-qPCR quantification of modified mRNA vaccines and therapeutics.
LC-MS/MS Quantification of Lipids in mRNA Delivery Systems
LNPs are the dominant delivery system, typically composed of ~50% ionizable cationic lipid, 10% neutral lipid, 38-40% cholesterol, and PEG-lipid. These ~100 nm particles mimic cell membranes, with cationic lipids complexing negatively charged mRNA for efficient encapsulation and endosomal escape. Analyzing cationic lipids via LC-MS/MS faces challenges:
Low polarity, causing difficulty in LC elution.
Peak tailing and high carryover.
Sensitivity loss upon peak shape optimization.
Optimization strategies include:
Selecting appropriate chromatographic columns for compound separation.
Optimizing mobile phase composition, additive concentration, and strong acid/weak acid ratios to improve peak shape, signal, and carryover.
Adjusting elution solvent composition/ratio to reduce carryover.
A robust method was developed with an LLOQ of 0.1 ng/mL for cationic lipids, carryover <0.1%, and a linear range spanning 3000-fold, suitable for diverse matrices.
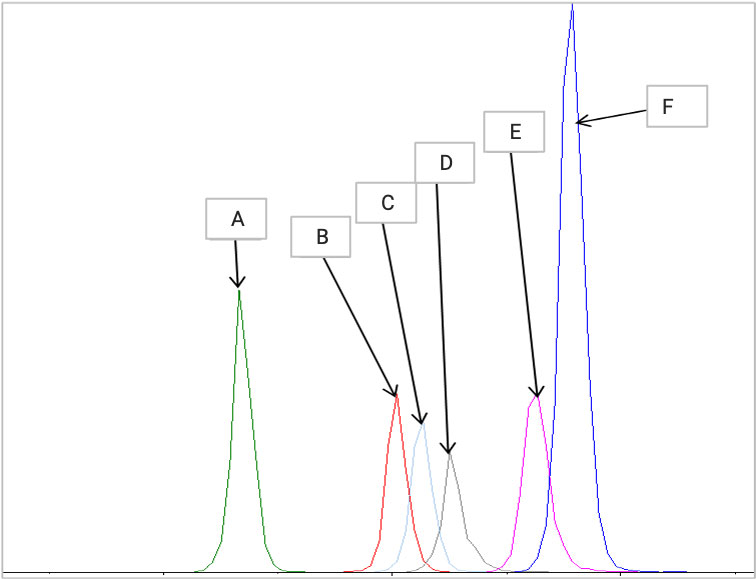
Figure 6. Simultaneous analysis of multiple cationic lipids.
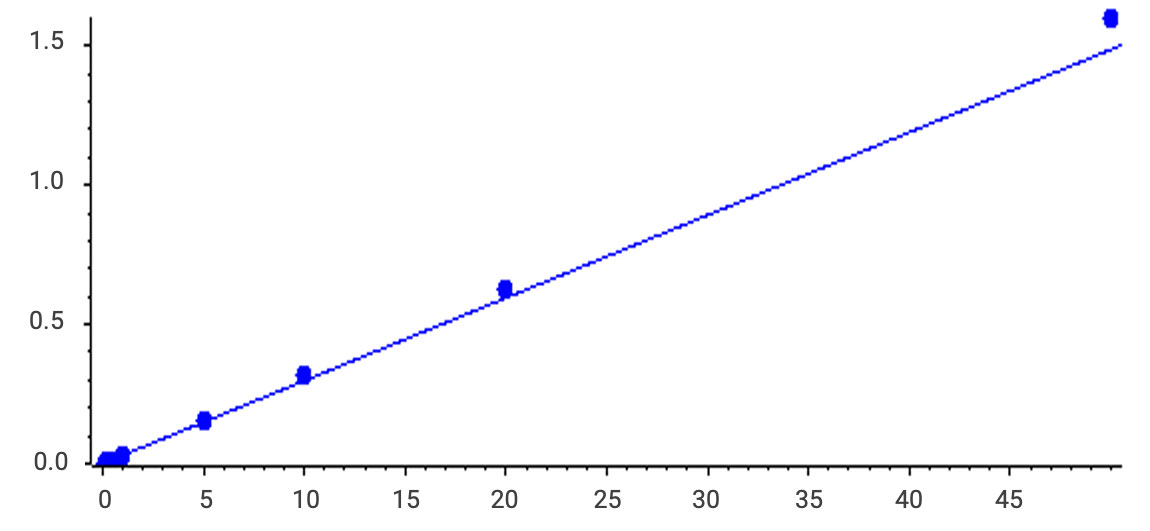
Figure 7. Cation lipid linearity (0.1- 50ng/mL, r=0.99498).
mRNA Capping Efficiency Assay in Modified mRNAs Using LC-HRAM
The 5' cap is critical for mRNA translation. Anti-reverse cap analogs (ARCAs, e.g., 3-O-Me-m7G(5')ppp(5')G) ensure correct cap orientation during transcription. However, competition with GTP prevents 100% capping. Liquid chromatography coupled with high-resolution accurate mass spectrometry (LC-HRAM) distinguishes and quantifies capped vs. uncapped mRNAs. WuXi AppTec DMPK validated an LC-HRAM method for mRNA capping efficiency assay of a 36-nucleotide (nt) mRNA.
A 36-nt ARCA-capped mRNA was transcribed in vitro using the HiScribe T7 ARCA mRNA Kit (NEB, MA) from linearized plasmid, purified with TURBO DNase (Thermo Fisher, NY) and Monarch RNA Cleanup Kit (NEB, MA), and stored at -80°C for LC-HRAM analysis.
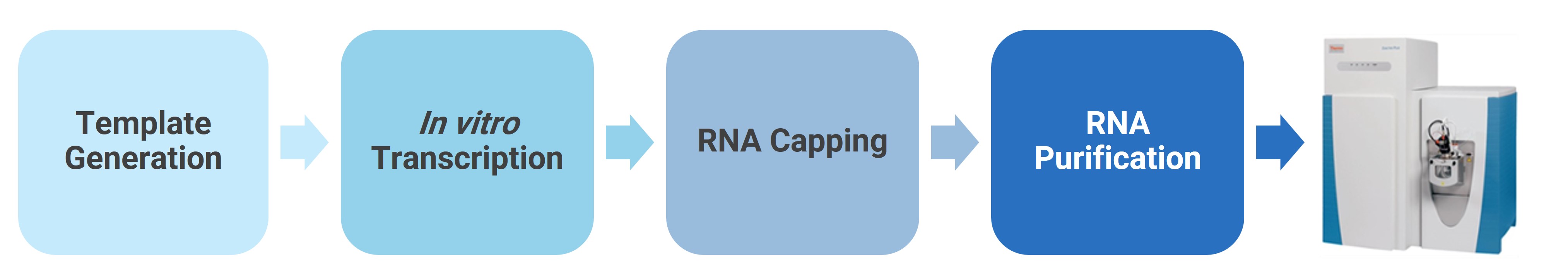
Figure 8. mRNA sample preparation and analysis protocols at WuXi AppTec DMPK.
Ion-pair reversed-phase (IP-RP) LC-MS with diisopropylethylamine (DIPEA) as the ion-pairing reagent and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as buffer enhances the separation of oligonucleotides. Chromatographic conditions were tested using ACQUITY UPLC Oligonucleotide BEH C18 or ACQUITY UPLC BEH C4 columns. While the C18 column enabled better separation with longer gradients, the C4 column achieved adequate characterization of the capped mRNA within 5 minutes without sacrificing resolution of lower-abundance species.
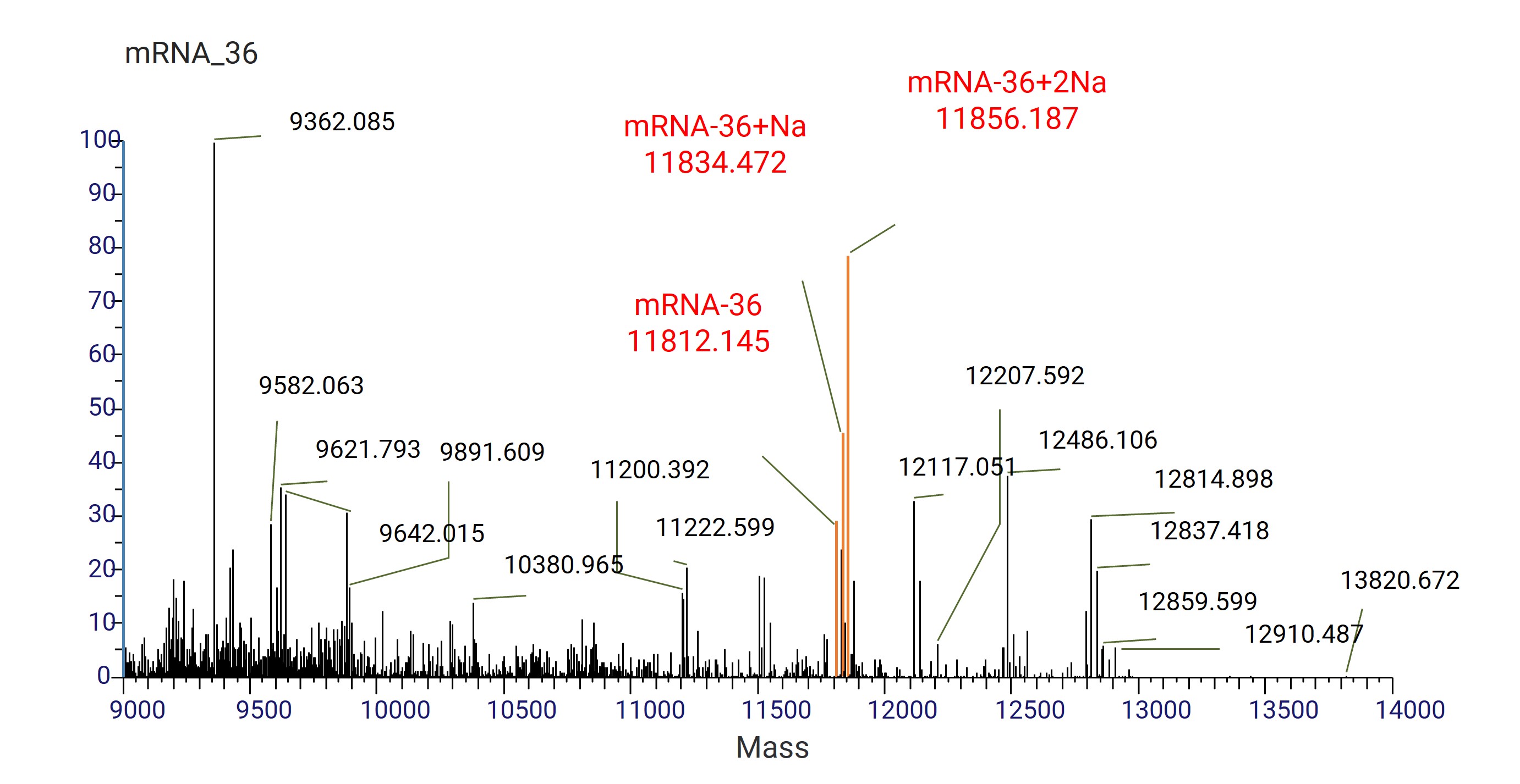
Figure 9. mRNA-36 deconvolution mass spectrometry.
The 36-nt ARCA-capped transcript (starting with 3-O-Me-m7G(5')ppp(5')G) was distinguished from uncapped transcripts (starting with ppp(5')G) based on mass and retention time differences via IP-RP LC-HRAM. Capping efficiency was calculated by quantifying capped and uncapped species.
RT-qPCR Quantification of Modified mRNA Vaccines and Therapeutics
Current mRNA analysis methods include RT-qPCR, cellular imaging, microarrays, LC-MS, and capillary gel electrophoresis. Due to its high sensitivity, specificity, selectivity, and ease of use, RT-qPCR is the gold standard for nucleic acid quantification and is widely used in research, diagnostics, forensics, and environmental testing. Key advantages:
Real-time monitoring of PCR amplification.
Precise quantification of starting material via cycle threshold (Ct) values.
Wide dynamic range.
Closed-tube format eliminates post-PCR processing.
RT-qPCR Method Development Flow
The RT-qPCR method development process includes sample lysis and mRNA extraction, primer design, reverse transcription, RT-qPCR, and data quantification.
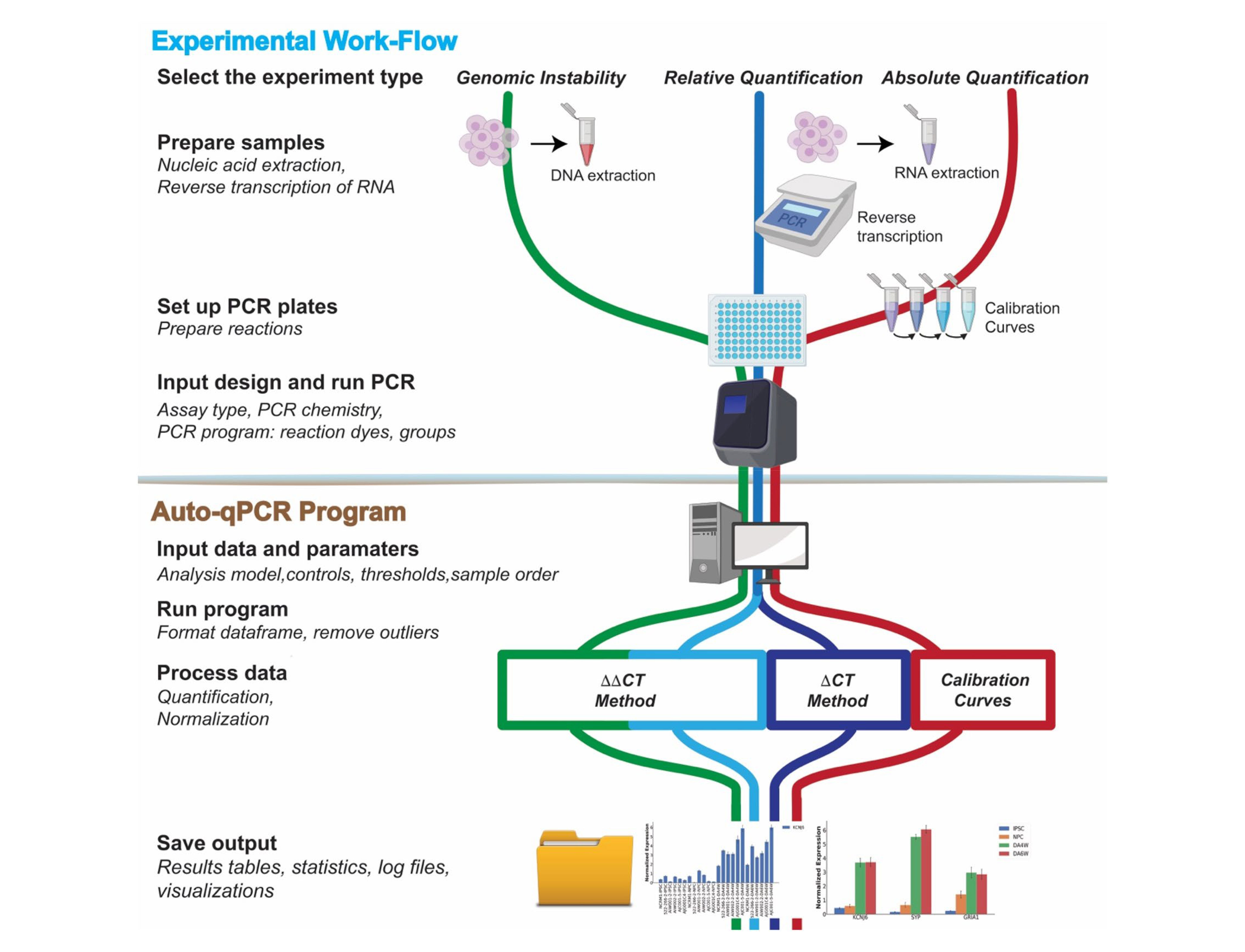
Figure 10. RT-qPCR workflow [7].
In RT-qPCR, one-step and two-step methods are the two most commonly used RT-qPCR methods. The one-step method can complete the first-strand cDNA synthesis reaction and the real-time PCR reaction in the same tube. The two-step method consists of two independent reactions, with first-strand cDNA synthesis followed by PCR amplification of the cDNA resulting from the first step.
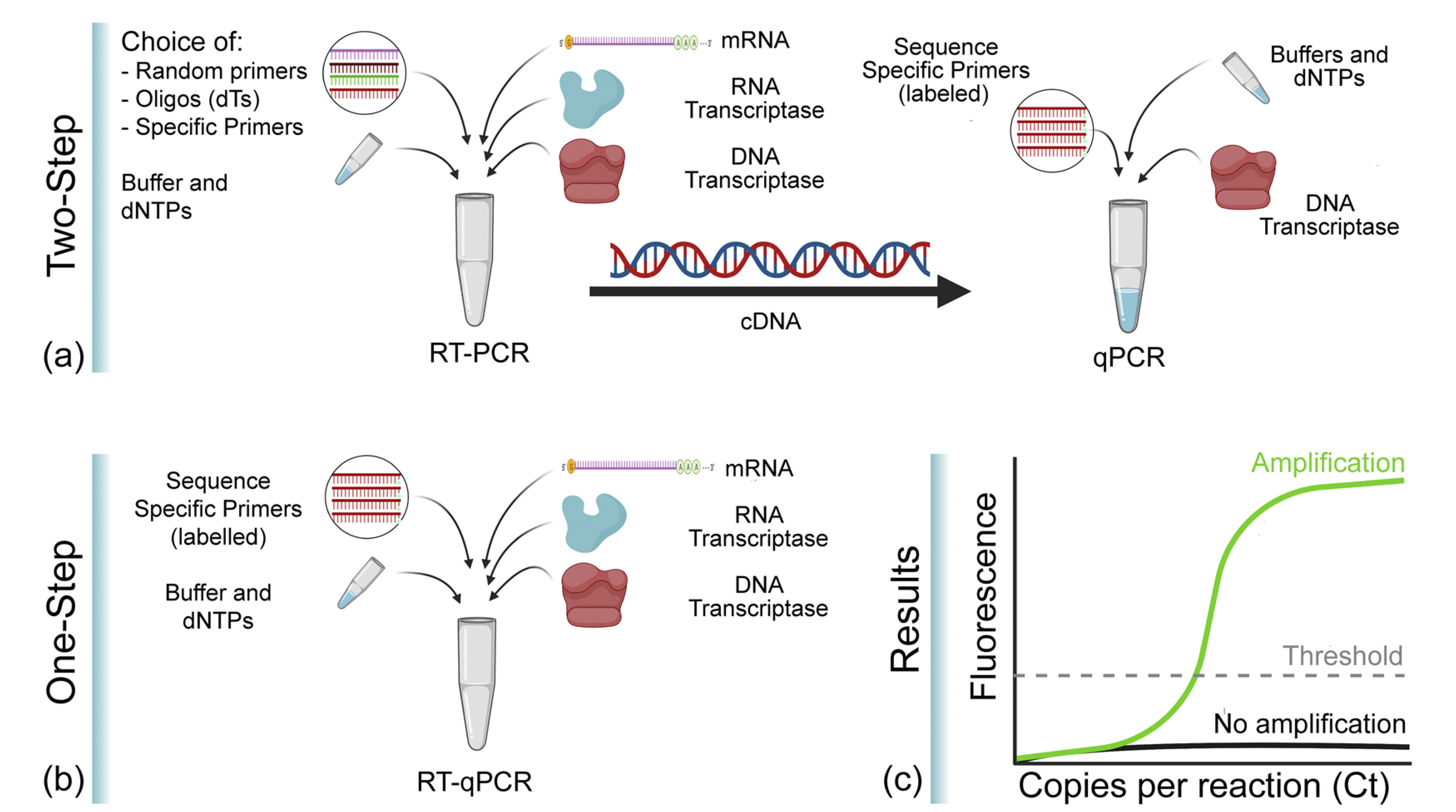
Figure 11. Comparison of RT-qPCR one-step and two-step procedures[8].
WuXi AppTec DMPK has developed an ultra-high detection sensitivity RT-qPCR method for the quantitative analysis of mRNA in multiple matrices such as mouse plasma, lung, brain, and tumor, and has demonstrated the specificity, selectivity, sensitivity, linear range, accuracy, and precision of the method. The method uses a kit to extract mRNA and then detect it in a one-step qPCR reaction based on TaqMan probes with good accuracy and precision.
This method achieves outstanding sensitivity (LLOQ = 0.001 ng/mL) and a broad dynamic range (0.001-500 ng/mL), with amplification efficiency between 90%-110%.
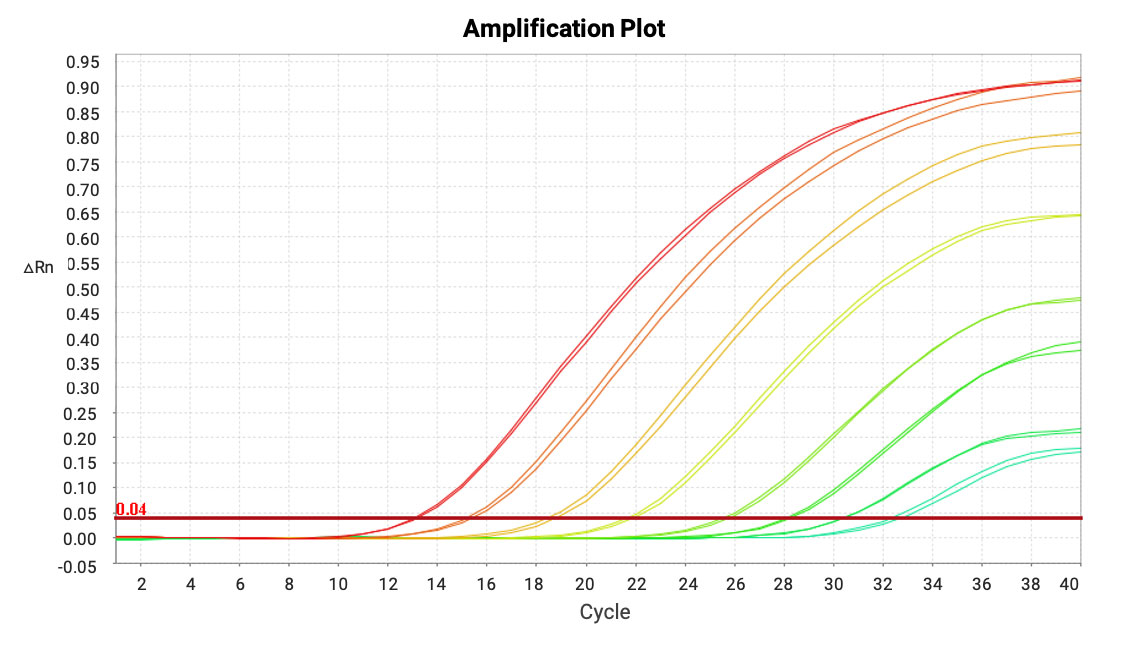
Figure 12. Amplification curves of mRNA in mouse lung homogenate samples.
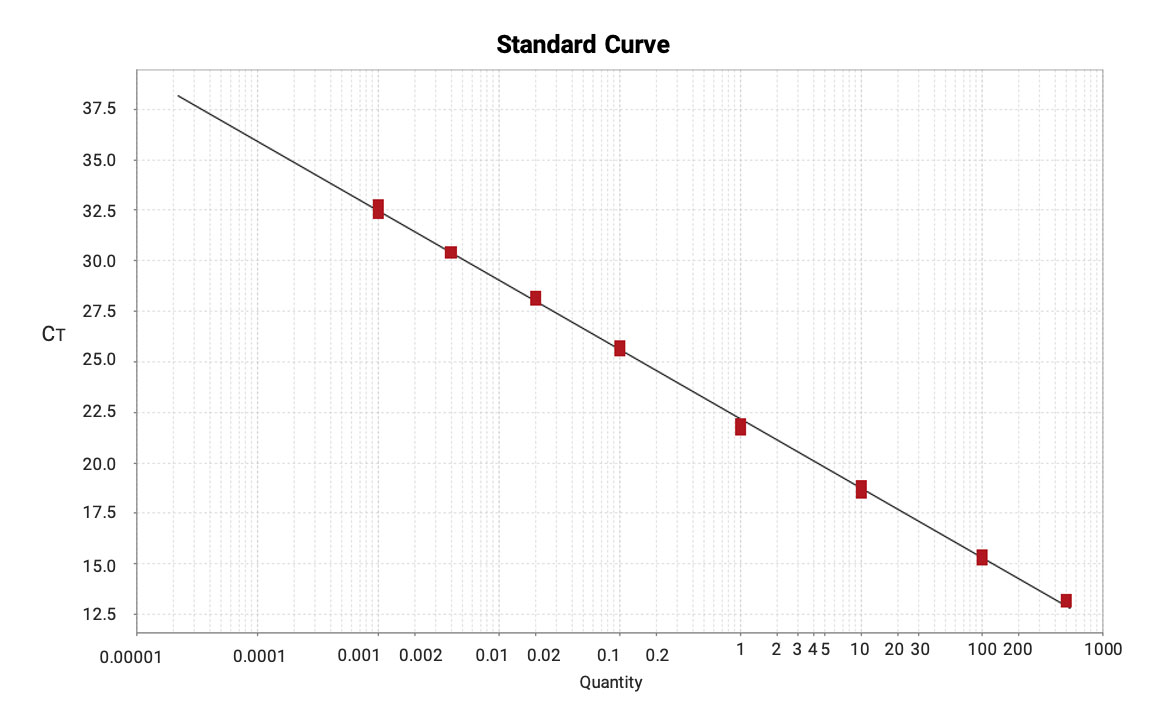
Figure 13. Calibration curve of mRNA in mouse lung homogenate samples (0.001-500 ng/mL, R2=0.999).
The study of mRNA modification and delivery has improved the stability and delivery efficiency of mRNA, making it have a broader application prospect. Quantitative analysis also provides more accurate mRNA capping rate, drug efficacy, accurate dosage, clinical research and efficacy evaluation, and other data support.
Concluding Remarks
We have established advanced analytical methodologies, including LC-MS/MS for mRNA delivery system characterization and high-resolution chromatography for 5' cap efficiency assessment. The integration of high-throughput platforms—such as QIAcube HT, KingFisher, and QuantStudio™ 7 Flex—enables rapid, large-scale sample analysis, significantly accelerating project timelines. Supported by expert teams specializing in nucleic acid analysis, primer design, and method development, WuXi AppTec DMPK streamlines workflows, addresses client-specific challenges, and expedites the advancement of mRNA vaccines and therapeutics into clinical applications.
Figure 14. High-throughput, automated nucleic acid extraction equipment at WuXi AppTec DMPK.
Authors: Wuyun Gong, Xianchun Zhang, Xiaoqing Huang, Jiaming Jiang, Zhiyu Li, Lili Xing
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Mingyuan Li,Yuan Li,Shiqin Li,Lin Jia,Haomeng Wang,Meng Li,Jie Deng,Ali Zhu,Liqiao Ma,Weihong Li,Peng Yu,Tao Zhu.The nano delivery systems and applications of mRNA. Eur J Med Chem. 2021 Oct 8;227:113910.
[2] Enyue Fang, Xiaohui Liu, Miao Li, Zelun Zhang, Lifang Song, Baiyu Zhu, Xiaohong Wu, Jingjing Liu, Danhua Zhao, and Yuhua Li.Advances in COVID-19 mRNA vaccine development.Signal Transduction and Targeted Therapy volume 7,Article number: 94 (2022).
[3] Shugang Qin,Xiaoshan Tang, Yuting Chen, Kepan Chen, Na Fan, Wen Xiao, Qian Zheng, Guohong Li, Yuqing Teng, Min Wu, Xiangrong Song.mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct Target Ther. 2022 May 21;7:166.
[4] Zhou Zhengjie, Li Xin. Research Progress on Messenger RNA Drug Modification and Its Delivery System, Journal of Zhejiang University, 2023 Aug 25; 52(4):439–450.
[5] Camilla Hald Albertsen, Jayesh A Kulkarni, Dominik Witzigmann, Marianne Lind, Karsten Petersson, Jens B Simonsen.The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022 Jul 3;188:114416.
[6] Nargish Parvin, Nargish Parvin, Tapas Kumar Mandal.Enhancing Vaccine Efficacy and Stability: A Review of the Utilization of Nanoparticles in mRNA Vaccines. Biomolecules 2024, 14(8), 1036.
[7] Gilles Maussion, Rhalena A.Thomas, Iveta Demirova, Gracia Gu, Eddie Cai, Carol X.‑Q.Chen,NargesAbdian,Theodore J. P. Strauss,Sabah Kelaï,Angela Nauleau‑Javaudin, Lenore K. Beitel,Nicolas Ramoz,Philip Gorwood&Thomas M. Durcan.Auto-qPCR: a python-based web app for automated and reproducible analysis of qPCR data. Scientific Reports volume 11, Article number: 21293 (2021) Cite this article.
[8] Maria Soler, Alexis Scholtz, Rene Zeto, Andrea M. Armani.Engineering photonics solutions for COVID-19.APL Photonics 5, 090901 (2020).
Related Services and Platforms




-

 DMPK BioanalysisLearn More
DMPK BioanalysisLearn More -

 Novel Drug Modalities DMPK Enabling PlatformsLearn More
Novel Drug Modalities DMPK Enabling PlatformsLearn More -

 Novel Drug Modalities BioanalysisLearn More
Novel Drug Modalities BioanalysisLearn More -

 Small Molecules BioanalysisLearn More
Small Molecules BioanalysisLearn More -

 Bioanalytical Instrument PlatformLearn More
Bioanalytical Instrument PlatformLearn More -

 PROTAC DMPK ServicesLearn More
PROTAC DMPK ServicesLearn More -

 ADC DMPK ServicesLearn More
ADC DMPK ServicesLearn More -

 Oligo DMPK ServicesLearn More
Oligo DMPK ServicesLearn More -

 PDC DMPK ServicesLearn More
PDC DMPK ServicesLearn More -

 Peptide DMPK ServicesLearn More
Peptide DMPK ServicesLearn More -

 mRNA DMPK ServicesLearn More
mRNA DMPK ServicesLearn More -

 Covalent Drugs DMPK ServicesLearn More
Covalent Drugs DMPK ServicesLearn More
Stay Connected
Keep up with the latest news and insights.