The central nervous system’s unique defense mechanisms—the blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB)—establish stringent pharmacological and toxicological defenses through selective regulation. In clinical research [1], strategies for delivering CNS-targeted drugs primarily follow two technical pathways: systemic administration (requiring BBB penetration, e.g., oral or intravenous administration) and local administration (bypassing the BBB, e.g., nasal-brain pathway delivery, intrathecal, or intraventricular administration) [2,3]. WuXi AppTec DMPK has overcome traditional technical limitations by developing a specialized intrathecal delivery system. Using a customized guidewire-guided intrathecal catheter, this system achieves precise drug delivery via lumbar intrathecal catheter placement in rodent and large animal models, enhancing the operational success rate to 100%*—significantly outperforming conventional direct lumbar puncture. This ensures direct drug delivery to CNS targets while minimizing systemic exposure risks and guarantees high data consistency by eliminating operational variability. In addition, it provides a standardized, highly reliable technical framework for preclinical CNS drug development. This article focuses on the rat intrathecal catheter, exploring strategies for high-quality intrathecal drug delivery. For large animal intrathecal studies, please refer to our previous blogs:
4 Best Practices for Intrathecal Administration in CNS Drug Development
Intrathecal Antisense Oligonucleotides: Pharmacokinetic Characteristics and Study Strategies
*Note: In internal validation experiments involving 80 rats and 57 monkeys, successful drug delivery was confirmed via CSF drug concentration measurements.
Key Challenges in Intrathecal Injection for CNS Drugs
Intrathecal administration involves direct drug delivery into the subarachnoid space (cerebrospinal fluid circulation system), bypassing the BBB to enable rapid CNS action. Clinically, this technique is applied in pain management, oncology, CNS infection interventions, and neurodegenerative disease therapy. However, its preclinical implementation demands precise anatomical targeting and dose control within confined spaces, relying heavily on specialized technical expertise. In preclinical research, rodents (e.g., rats, mice) and non-human primates (NHPs) serve as primary animal models. However, translational challenges arise from interspecies differences in anatomy (e.g., vertebral spacing, CSF volume gradients), technical precision, species-specific drug metabolism (e.g., enzyme activity, barrier permeability), and ethical considerations (e.g., 3R principles, patient rights), underscoring the complexity of cross-species research (Table 1).
Table 1. Comparative Analysis of Intrathecal Administration in Rats, Monkeys, and Humans [4-8].
Parameter | Rat | Monkey (NHP) | Human |
Vertebral Spacing | Narrow lumbar interspace (L4-L6: 1-2 mm), high spinal cord injury risk | Wider lumbar interspace (L4-L6: 3-5 mm) | Wide lumbar interspace (L3-L4: 8-10 mm), ample puncture space |
CSF Volume | ~200-500 µL, strict volume control required | ~10-15 mL, intermediate between rats and humans | ~150 mL, tolerates larger volumes |
Drug Diffusion Kinetics | Rapid CSF turnover (29 cycles /24 h), high drug clearance | Moderate turnover (4 cycles /24 h), closer to human PK profiles | Slow turnover (5 cycles /24 h), prolonged drug action |
Species Metabolism | High metabolic enzyme activity, short drug half-life | Metabolic pathways are highly similar to humans | Significant inter-individual metabolic variability |
BBB Permeability | Immature BBB, potential overestimation of CNS drug penetration | BBB structure closely resembles humans, and reliable data extrapolation | Intact BBB limits systemic drug diffusion into the CNS |
Translational Utility | Efficient for mechanistic studies, limited clinical predictability | Bridge model for validating complex therapies | Gold standard for efficacy/safety, constrained by ethics and cost |
Cost | Low | High | Highest |
The rat intrathecal delivery is a powerful tool for studying CNS diseases and drug candidates. However, its technical complexity and operational risks demand extensive anatomical knowledge and skilled execution to ensure animal welfare, scientific rigor, and data reliability.
Evolution of Rat Intrathecal Delivery: From Intrathecal Injection to Intrathecal Catheter
Rat intrathecal delivery methods are sorted out into two categories, each of which has distinct challenges as below:
Direct Puncture: Relies on blind puncture (L4-L5 /L5-L6 localization), where success largely depends on operator experience. Repeated attempts have a high risk of paravertebral muscle trauma, epidural hematoma, or spinal cord injury.
Intrathecal Catheter: Uses pre-implanted microcatheters for precise delivery. However, long-term catheterization may lead to thrombosis, subarachnoid granuloma, or bacterial infection.
To confirm successful drug delivery into the subarachnoid space (vs. epidural or intravascular misplacement), behavioral responses (e.g., tail-flick reflex) or physiological indicators (e.g., CSF sampling) are employed [9]. Intrathecal cannulation is preferred for preclinical studies due to reduced animal stress, minimized tissue damage from repeated punctures, and lower postoperative complications (e.g., urinary retention, motor dysfunction, infection).
Optimization of intrathecal catheterization focuses on catheter placement, selection, and fixation. WuXi AppTec DMPK has refined a streamlined cannulation method (Figure 1) enabling single-operator procedures, shorter surgery times, high post-operative catheter patency, and support for chronic/repeat dosing. Postoperative monitoring showed stable body weights (Figure 2) and no pain-related abnormalities (Figure 3).

Figure 1. Schematic of WuXi AppTec DMPK’s Optimized Rat Intrathecal Catheterization Model.

Figure 2. Pre- and Post-Operative Body Weight Changes (4-Day Monitoring, N=5).

Figure 3. Post-Operative Pain Assessment (Hindlimbs, N=5).
How to Increase Intrathecal Injection/Catheterization Success Rate
Using pemetrexed as a test compound, this study systematically compared direct puncture and intrathecal catheter in terms of brain drug exposure [11]. A catheterization-based model was further used to evaluate critical parameters—administration volume (V), infusion rate (R), and duration (T)—establishing safety thresholds (Table 2; animal strain: SD /Male /N=5, dose: 1 mg/kg; results in Figures 4-6, Tables 3-5).
Table 2. Intrathecal Injection Validation Study Design.
Group | Surgery | Volume (µL) | Administration | Time Points (h) | Sample Collection |
1 | NA | 100 | Intrathecal | 0.75, 2, 4, 6, 10, 24 | Blood (jugular vein), CSF (cisterna magna) |
2 | NA | 300 | Intravenous | ||
3 | Intrathecal Catheter | 100 | Bolus | ||
4 | Intrathecal Catheter | 100 | Infusion (20 min) | ||
5 | Intrathecal Catheter | 30 | Infusion (20 min) | ||
6 | Intrathecal Catheter | 50 | Infusion (20 min) | ||
7 | Intrathecal Catheter | 70 | Infusion (20 min) |
Effect of different administration routes on drug exposure in the brain (G1-G3 comparison, shown in Figure 4 and Table 3).
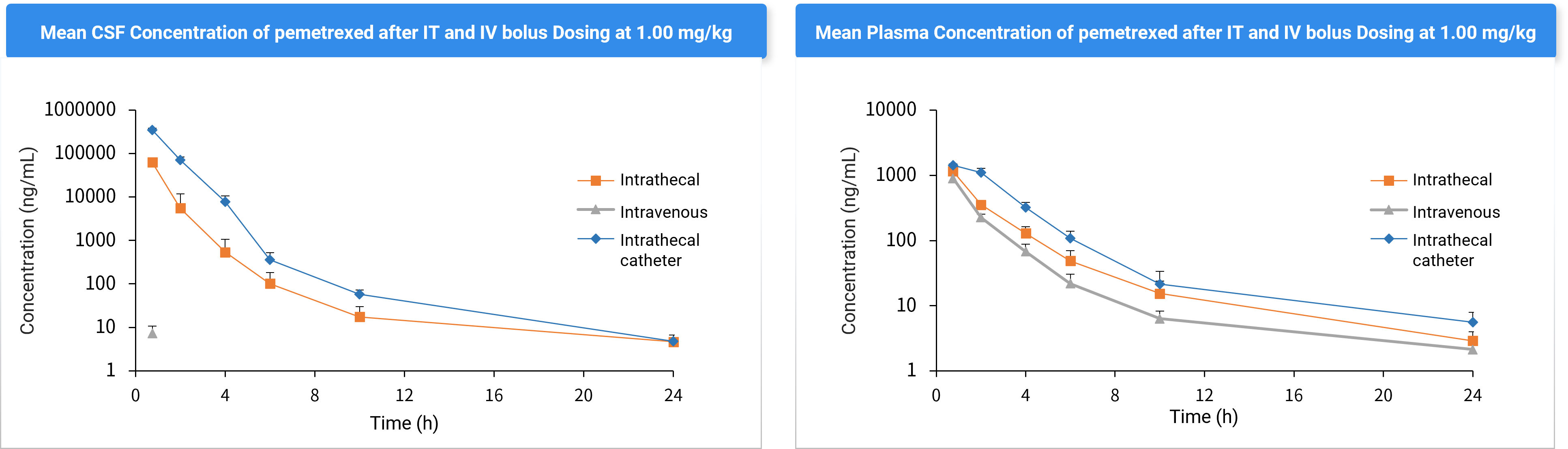
Figure 4. CSF and Plasma Concentrations after Intrathecal Injection (G1), IV (G2), and Intrathecal Catheterization (G3).
Table 3. Pharmacokinetic (PK) Parameters of Pemetrexed in Plasma and CSF After Intrathecal Injection (G1), Intravenous Injection (G2), and Intrathecal Catheterization (G3) (Mean ± SD).
Parameters | Intrathecal (G1) | Intravenous (G2) | Cannulation (G3) | Intrathecal (G1) | Intravenous (G2) | Cannulation (G3) |
Plasma | CSF | |||||
Cmax (ng/mL) | 1151 ± 186 | / | 1425 ± 67.1 | 62532 ± 14328 | ND | 349585 ± 36807 |
Tmax (h) | 0.75 ± 0.0 | / | 0.75 ± 0.0 | 0.75 ± 0.0 | ND | 0.75 ± 0.0 |
AUC0-24 (ng.h/mL) | 2098 ± 232 | 2109 ± 388 | 4138 ± 567 | 56154 ± 22992 | ND | 410045 ± 45696 |
ND = Not determined | ||||||
Conclusion: Pemetrexed cannot penetrate the blood-brain barrier via systemic administration. Intrathecal catheter significantly enhances CSF exposure compared to non-catheter intrathecal injection, supporting catheterization as the preferred method for data consistency.
Impact of Infusion Rate on CNS Drug Exposure
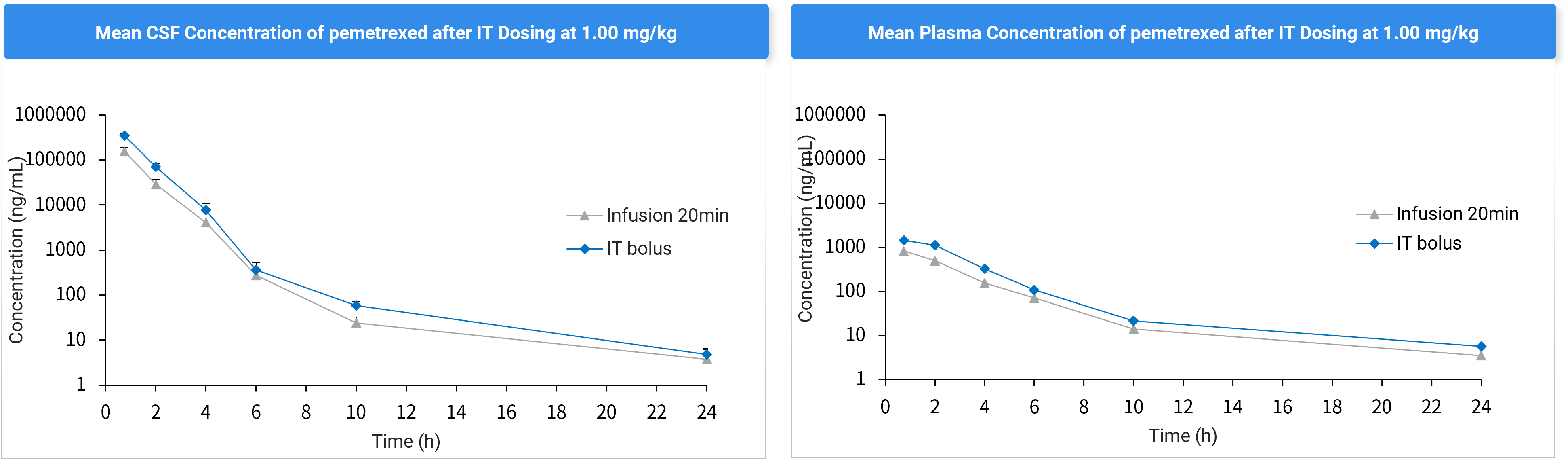
Figure 5. CSF and Plasma Concentration Profiles After Bolus (G3) vs. 20-Minute Infusion (G4) via Intrathecal Catheterization.
Table 4. PK Parameters of Pemetrexed in Plasma and CSF After Bolus (G3) vs. 20-Minute Infusion (G4) (Mean ± SD).
Parameters | Infusion (20min) | Bolus | Infusion (20min) | Bolus |
Plasma | CSF | |||
Cmax (ng/mL) | 774 ± 165 | 1425 ± 67.1 | 132580 ± 64868 | 349585 ± 36807 |
T1/2 (h) | 3.54 ± 0.55 | 3.58 ± 0.42 | 2.87 ± 1.33 | 3.15 ± 0.51 |
AUC0-last (ng.h/mL) | 1944 ± 405 | 4138 ± 567 | 152352 ± 71179 | 410045 ± 45696 |
Conclusion: For pemetrexed, slower infusion (20 min) achieves lower CNS exposure compared to bolus injection, which is likely due to its rapid clearance kinetics.
Impact of Administration Volume on CNS Drug Exposure

Figure 6. CSF and Plasma PK Profiles at Different Administration Volumes (30–100 µL, G4-G7).
Table 5. PK Parameters of Pemetrexed in Plasma and CSF at Different Administration Volumes (30–100 µL, G4-G7) (Mean ± SD).
Parameters | Dose volume(µL) | |||||||
30 | 50 | 70 | 100 | 30 | 50 | 70 | 100 | |
Plasma | CSF | |||||||
Cmax (ng/mL) | 533 ± 152 | 588 ± 84.9 | 692 ± 119 | 774 ± 165 | 54818 ± 38018 | 87344 ± 73687 | 136951 ± 26034 | 132580 ± 64868 |
T1/2 (h) | 5.58 ± 2.56 | 3.90 ± 0.57 | 3.03 ± 0.065 | 3.54 ± 0.55 | 2.32 ± 1.20 | 2.02 ± 0.45 | 2.72 ± 0.81 | 2.87 ± 1.33 |
AUC0-last (ng.h/mL) | 1768 ± 448 | 1607 ± 642 | 2122 ± 453 | 1944 ± 405 | 74723 ± 48309 | 125705 ± 96715 | 175802 ± 49515 | 152352 ± 71179 |
Conclusion: Smaller volume (30 µL) compromises dosing accuracy due to catheter dead space, while larger volume (100 µL, ~1 /5 of total CSF volume) risks intrathecal pressure elevation and neurobehavioral anomalies. Recommended optimal volume: <100 µL to balance precision and physiological tolerance.
Case Study: Nusinersen Delivery via Rat Intrathecal Cannulation
Animal model: SD /Male /N=5, dosage: 0.67 mg/kg
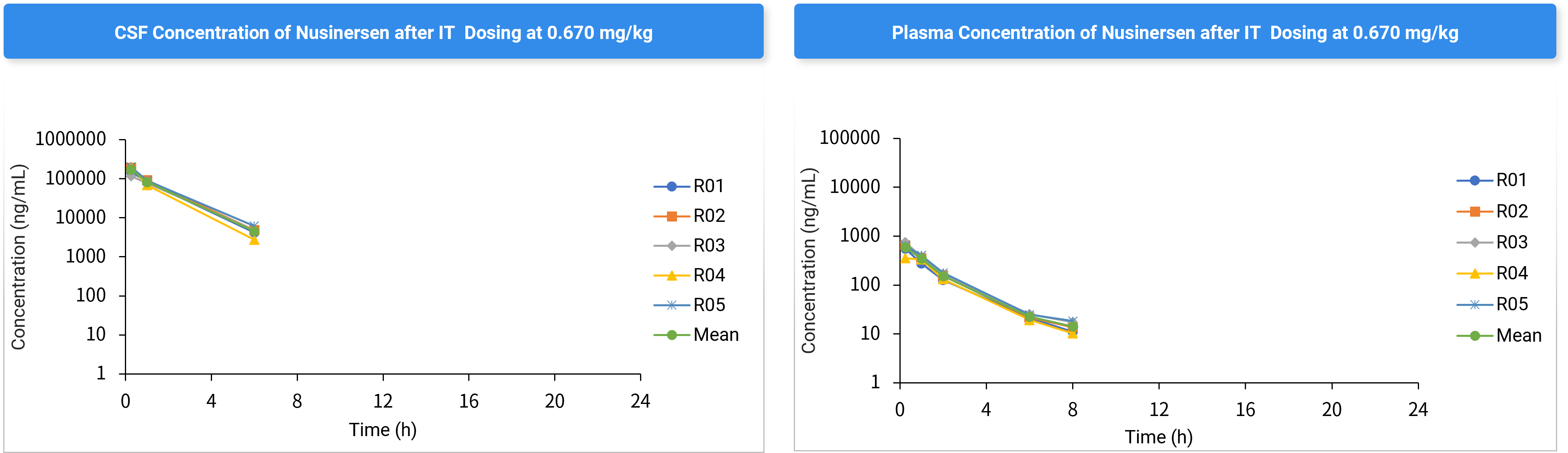
Figure 7. Nusinersen Concentration-Time Profiles in CSF and Plasma.
Table 6. Nusinersen CSF Concentration-Time Data (Mean ± SD).
CSF concentration (ng/mL) | |||||||||
Time (h) | R01 | R02 | R03 | R04 | R05 | Mean | ± | SD | CV (%) |
0.25 | 149000 | 190000 | 117000 | 202000 | 202000 | 172000 | ± | 37676 | 21.9 |
1 | 83400 | 91600 | 78000 | 68200 | 89000 | 82040 | ± | 9348 | 11.4 |
6 | 4160 | 4800 | 4840 | 2760 | 6160 | 4544 | ± | 1234 | 27.2 |
24 | BQL | BQL | BQL | BQL | BQL | ND | ± | ND | ND |
Conclusion: Optimized infusion rates and volumes significantly enhance CNS drug delivery while minimizing physiological interference. This intrathecal catheter model demonstrates robust applicability for CNS-targeted therapies.
Final Remarks
This study provides a systematic framework for optimizing intrathecal injection/catheter systems, offering critical insights for CNS drug development. WuXi AppTec DMPK’s validated rat intrathecal cannulation model enables precise, reproducible preclinical CNS research, accelerating the translation of CNS therapeutics to clinical trials.
Authors: Furong Jiao, Tietu Li, Li Wang, Cheng Tang
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Tumani H, Huss A, Bachhuber F. The cerebrospinal fluid and barriers - anatomic and physiologic considerations. Handb Clin Neurol. 2017;146:21-32.
[2] Fowler MJ, Cotter JD, Knight BE, Sevick-Muraca EM, Sandberg DI, Sirianni RW. Intrathecal drug delivery in the era of nanomedicine. Adv Drug Deliv Rev. 2020;165-166:77-95.
[3] Nance E, Pun SH, Saigal R, Sellers DL. Drug delivery to the central nervous system. Nat Rev Mater. 2022 Apr;7(4):314-331. doi: 10.1038/s41578-021-00394-w.
[4] Mazur C, Fitzsimmons B, Kamme F, Nichols B, Powers B, Wancewicz E. Development of a simple, rapid, and robust intrathecal catheterization method in the rat. J Neurosci Methods. 2017 Mar 15;280:36-46.
[5] Khani M, Fu AQ, Pluid J, Gibbs CP, Oshinski JN, Xing T, Stewart GR, Zeller JR, Martin BA. Intrathecal catheter implantation decreases cerebrospinal fluid dynamics in cynomolgus monkeys. PLoS One. 2020 Dec 30;15(12):e0244090.
[6] Shafique S, Rayi A. Anatomy, Head and Neck, Subarachnoid Space. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: http://www.ncbi.nlm.nih.gov/books/NBK557521/
[7] Jaumard NV, Leung J, Gokhale AJ, Guarino BB, Welch WC, Winkelstein BA. Relevant Anatomic and Morphological Measurements of the Rat Spine: Considerations for Rodent Models of Human Spine Trauma. Spine (Phila, Pa 1976). 2015 Oct 15;40(20):E1084-92.
[8] Vuillemenot BR, Korte S, Wright TL, Adams EL, Boyd RB, Butt MT. Safety Evaluation of CNS Administered Biologics-Study Design, Data Interpretation, and Translation to the Clinic. Toxicol Sci. 2016 Jul;152(1):3-9.
[9] Chen Y, Mazur C, Luo Y, Sun L, Zhang M, McCampbell A, Tomassy GS. Intrathecal Delivery of Antisense Oligonucleotides in the Rat Central Nervous System. J Vis Exp. 2019 Oct 29;(152).
[10] Zheng Y, et al. Comparison of different methods for drug delivery via the lumbar spinal subarachnoid space in rats. JSouthMedUniv,2019,39(10):1246-1252.
[11] Sun JM, Nam MH, Chung JY, Im B, Lee SY, Suh YL, Ahn JS, Park K, Ahn MJ. Safety and pharmacokinetics of intrathecal administration of pemetrexed in rats. Cancer Chemother Pharmacol. 2011 Aug;68(2):531-8.
Related Services and Platforms




-

 In Vivo PharmacokineticsLearn More
In Vivo PharmacokineticsLearn More -

 Therapeutic Areas DMPK Enabling PlatformsLearn More
Therapeutic Areas DMPK Enabling PlatformsLearn More -

 Rodent PK StudyLearn More
Rodent PK StudyLearn More -

 Large Animal (Non-Rodent) PK StudyLearn More
Large Animal (Non-Rodent) PK StudyLearn More -

 Clinicopathological Testing Services for Laboratory AnimalsLearn More
Clinicopathological Testing Services for Laboratory AnimalsLearn More -

 High-Standard Animal Facilities and Animal WelfareLearn More
High-Standard Animal Facilities and Animal WelfareLearn More -

 Preclinical Formulation ScreeningLearn More
Preclinical Formulation ScreeningLearn More
Stay Connected
Keep up with the latest news and insights.











