The payloads of antibody-drug conjugates (ADCs) are categorized into DNA alkylating agents, DNA topoisomerase inhibitors, microtubule disruptors, and RNA pol II inhibitors, among others. In addition to payloads with tumors as indications, payloads with other mechanisms of action, such as glucocorticoids, expand ADC applications to inflammatory and autoimmune diseases. Currently marketed ADCs employ a limited number of payloads, primarily including MMAE, DM1, SN-38, PBD, DXd, and their derivatives etc. This overview focuses on the mechanisms, absorption, distribution, metabolism, and excretion (ADME), and drug interactions (DDIs) properties of 8 common ADC payloads, and summarizes the pharmacokinetic profile of these 8 ADC payloads in a table at the end of this article.
ADC Payload Class 1: Auristatin derivatives MMAE and MMAF
#1 MMAE (Monomethyl Auristatin E)
MMAE is a potent cytotoxic agent that binds to microtubules, inhibiting cell division and inducing apoptosis. It has high permeability and exhibits a strong bystander effect [1].
Distribution:
[3H]-MMAE showed species-dependent plasma protein binding in ICR mice (18.8%-28.5%), Wistar rats (72.9%-73.5%), cynomolgus monkeys (17.1%-18.9%), and humans (67.9%-82.2%) (Table 1). Binding in rats and monkeys remained constant across concentrations (1–100 nmol/L), whereas mice and humans displayed concentration-dependent variability. Human whole blood-to-plasma ratios ranged from 0.79 to 0.98[2].
Table 1. Plasma Protein Binding Value of MMAE in Mouse, Rat, Cynomolgus, and Human[3].
Species | Concentration | ||
1 nmol/L | 10 nmol/L | 100 nmol/L | |
Mouse | 18.8% | 19.6% | 28.5% |
Rat | 72.9% | 73.5% | 72.0% |
Cynomolgus | 17.1% | 17.8% | 18.9% |
Human | 67.9% | 77.5% | 82.2% |
In MDA-MB-468 tumor-bearing mice, MMAE plasma concentrations declined rapidly after intravenous (IV) administration (0.1 mg/kg), with only 0.3% of the dose remaining at 5 minutes post-dose. Plasma AUC0–12h and AUC0–last were 54.3 and 54.5 ng·h/mL, respectively. Systemic clearance (CL) was 60 mL/h, terminal half-life (t1/2) was 2.5 h, and steady-state volume of distribution (Vdss) was 42 mL. Tissue concentrations declined slowly, with a 50% reduction by 168 h, MMAE was mainly distributed in the liver[4].
Radioactivity in whole blood and plasma of male and female Sprague Dawley (SD) rats after intravenous injection of 0.06 mg/kg of [3H]-MMAE is shown in Figure 1[3].
![Radioactivity Curves in Whole Blood and Plasma of Male (Left) and Female (Right) SD Rats After Intravenous Injection of 0.06 mg/kg of [3H]-MMAE Radioactivity Curves in Whole Blood and Plasma of Male (Left) and Female (Right) SD Rats After Intravenous Injection of 0.06 mg/kg of [3H]-MMAE](https://wuxiapptec-dmpkcatalog-prod.oss-cn-shanghai.aliyuncs.com/Public/Uploads/ueditor/upload/image/20250521/1747817327812370.jpg)
Figure 1. Radioactivity Curves in Whole Blood and Plasma of Male (Left) and Female (Right) SD Rats After Intravenous Injection of 0.06 mg/kg of [3H]-MMAE.
Metabolism:
MMAE was primarily metabolized by CYP3A4, with minor contributions from CYP2D6. 13 metabolites were identified in preclinical and clinical studies (Figure 2), including demethylation, hydroxylation, amide hydrolysis, and oxidation. After [3H]-MMAE was incubated at 10μM for 240 minutes, 12 metabolites were detected from monkey hepatocytes. These 12 metabolites can all be detected in the monkey hepatocytes, 9 of which were detected in rat hepatocytes, and a different set of 9 were seen in human hepatocytes. Among the three species, the signals of M4 (O-demethylation) and M6 (hydroxylation) were relatively strong, suggesting that they were the main metabolites. No specific metabolites were detected in the human species. M4 and M7 showed reduced cytotoxicity compared to MMAE, while M8 exhibited comparable toxicity, i.e., cytotoxic M7<< M4< M8 ≈ MMAE[3].
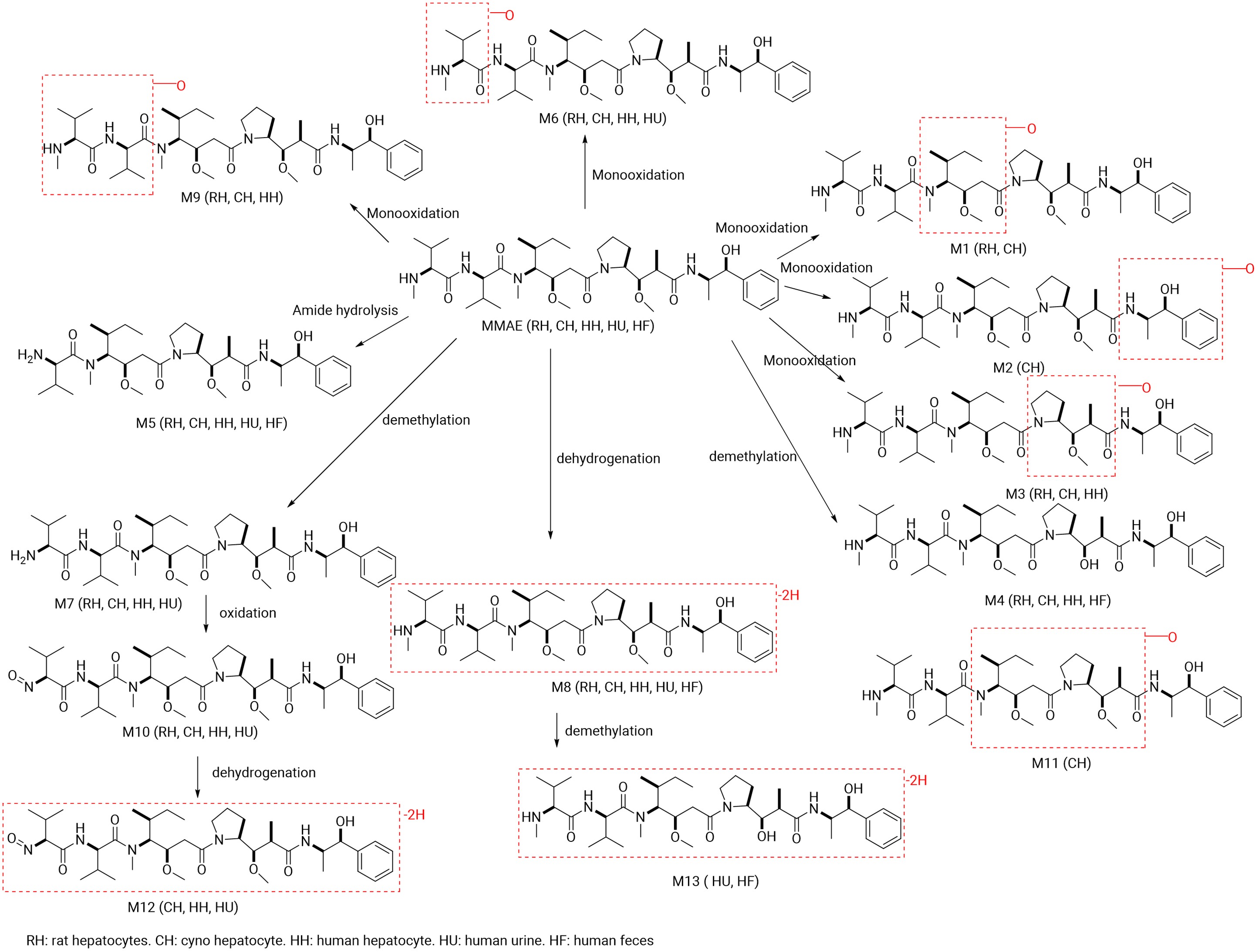
Figure 2. Summary of MMAE Metabolites Identified in In Vitro and Clinical Samples[3].
Excretion:
The rat excretion study showed that MMAE is secreted through bile, mainly excreted through feces, and a part is excreted through urine. In rats, 97% (male) and 102% (female) of the administered [3H]-MMAE was detected out in feces and 15% (male) and 9% (female) of the administered [3H]-MMAE was detected out in urine after single intravenous injection of [3H]-MMAE. Most of the radioactive products in rat urine and feces were prototypical [3H]-MMAE. A smaller peak could be detected in the feces and identified as the metabolite M4 (O-demethylation metabolite). Approximately 90-95% of [3H]-MMAE was excreted 48 hours post-dose, and the half-life of [3H]-MMAE in rats was 1-2.3 days. No radioactivity was detected in the rat carcasses 28 days post-dose, suggesting that MMAE and its metabolites had been completely excreted from the body. [5].
Drug Interactions:
MMAE did not induce CYP450 enzymes. It caused direct inhibition of CYP3A4/5 by midazolam 1’-hydroxylation (CYP3A-M) with an IC50 of 10μM and showed little or no direct inhibition of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP3A4/5 by testosterone 6β-hydroxylation (CYP3A-T), with IC50 of >100μM. MMAE acted as a time-dependent inhibitor of CYP3A4/5, with a Kinact value of 0.1 min-1, indicating that at saturating concentrations of MMAE, approximately 10% CYP3A4/5 activity would be inactivated per min. The KI of 1.12 µM indicates the concentration of MMAE that gave half the maximum rate of inactivation. In vitro studies indicate that MMAE is a substrate for P-glycoprotein (P-gp) but not for breast cancer resistance protein (BCRP), multidrug resistance-associated protein 2 (MRP2), organic cation transporter 2 (OCT2), organic anion transporters (OAT1, OAT3), or organic anion transporting polypeptides (OATP1B1, OATP1B3). At clinically relevant concentrations, MMAE did not inhibit P-gp, BCRP, bile salt export pump (BSEP), MRP2, OCT1, OCT2, OAT1, OAT3, OATP1B1, and OATP1B3 [5].
#2 MMAF (Monomethylauristatin F) [6]
MMAF disrupts microtubule networks, leading to cell cycle arrest and apoptosis in tumor cells. Its C-terminal carboxyl group forms more hydrogen bonds with microtubule proteins compared to MMAE, enhancing its binding capability. However, due to its poor membrane permeability, MMAF often fails to exert the bystander effect as an ADC payload. Blenrep used a non-cleavable linker to conjugate MMAF, releasing the linker-payload Cys-mcMMAF (see Figure 3).
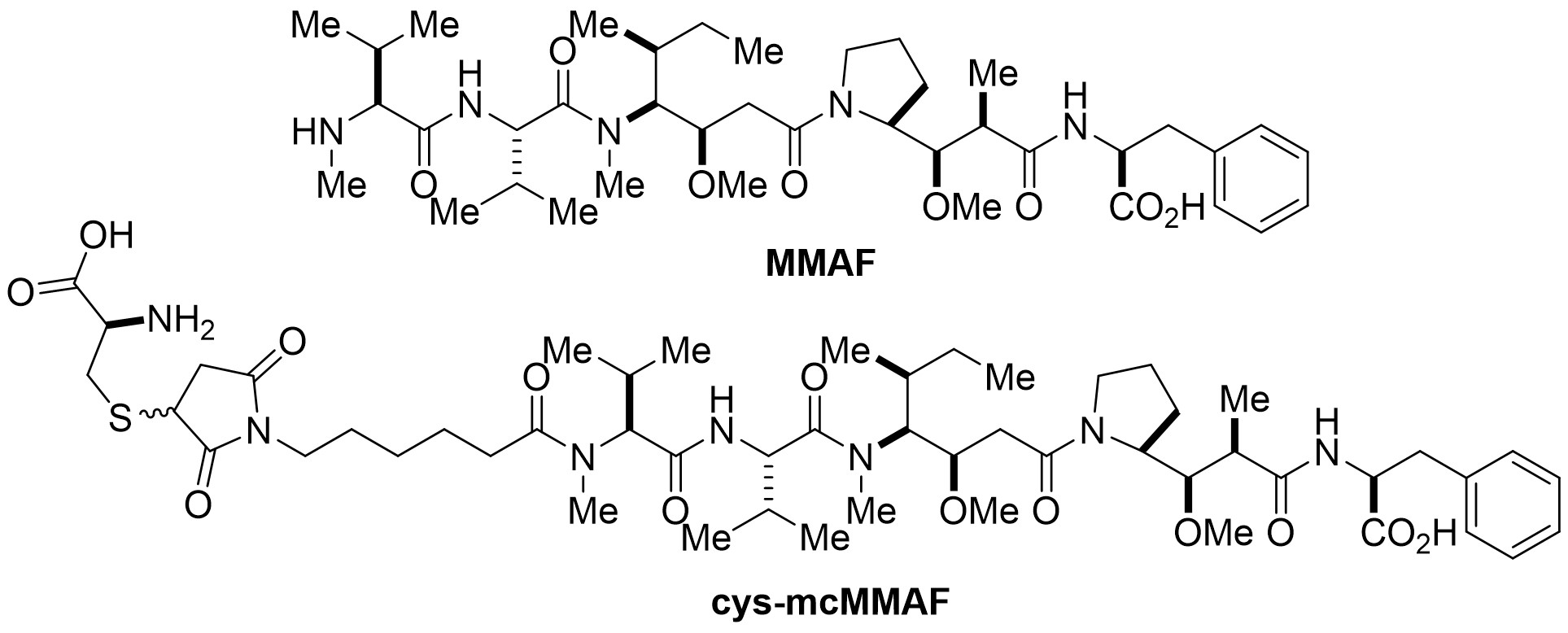
Figure 3. Structures of MMAF and Cys-mcMMAF.
Distribution:
Cys-mcMMAF exhibits concentration-dependent binding in human plasma: unbound percentage was 49.1%-61.8% at 0.5 ng/mL, 68.8%-70.7% at 5 ng/mL, and 80.9%-88.7% at 50 ng/mL.
Metabolism:
In vitro assay showed that cyclic cys-mcMMAF was formed from linear cys-mcMMAF through hydrolysis and dehydration, and this process does not rely on NADPH or UDPGA. After incubating 3H-Cys-mcMMAF with rat, monkey, and human liver S9 fractions, the metabolism primarily occurred through non-enzymatic catalysis, with minor oxidative and phase II metabolism.
Excretion:
After a single intravenous injection of 10 mg/kg 3H-Cys-mcMMAF in rats, most of the radioactive dose was excreted via feces (approximately 83%), while urinary excretion (about 13%) was the second excretion route. Radioactive material was rapidly eliminated, with a total recovery of 94% within 48 hours post-dose, 96% within 7 days post-dose. The primary component in urine was linear Cys-mcMMAF, and all other confirmed metabolites account for less than 1% of the radioactive dose, while the primary component in feces was cyclic Cys-mcMMAF, and all other confirmed metabolites account for less than 5% of the radioactive dose.
Drug Interactions:
In vitro studies revealed that Cys-mcMMAF did not act as an inhibitor or inducer of CYP450 enzymes. It was a substrate for OATP1B1, OATP1B3, MRP1, MRP2, MRP3, and BSEP, and may be a substrate for P-gp.
ADC Payload Class 2: Maytansine Derivatives DM1 and DM4
Meltansin is a macrolide of the ansamycin family, and DM1 and DM4 are derivatives of maytansin (see Figure 4 for structures). DM1 and DM4 inhibit microtubulin polymerization and assembly, leading to cell cycle arrest and apoptosis[7].
#3 DM1
Distribution:
The plasma protein binding of DM1 is 97.1% in rats, 91.5% in cynomolgus monkeys, and 92.5% in humans. These binding percentages do not show significant concentration dependence in the range of 20–1000 ng/mL [8].
Metabolism:
Enzyme phenotyping in liver microsomes revealed that DM1 metabolism is primarily NADPH-dependent, with CYP3A4 and CYP3A5 as the major enzymes involved. The results from human CYP450 recombinant enzyme phenotyping were consistent with liver microsome data, confirming CYP3A4 and CYP3A5 as the primary metabolic enzymes. CYP2C9 and CYP2E1 play minor roles, while other isoforms (CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C18, CYP2C19, CYP2D6, and CYP4A11) play little or no role.
Metabolites M2, M3, and M7 (structures in Figure 4) were identified in enzyme phenotyping studies. M3 and M2 should be more soluble than the parent compound. M7 may be less soluble and may be active.

Figure 4. The Structures of DM1/DM4 and Proposed Structures of Metabolites of DM1[8].
Drug Interactions:
DM1 showed no induction potential for CYP1A2, 2B6, or 3A4/5 at concentrations of 0.1, 1, 10, 100, 500, and 1000 nM. In NADPH-generating systems, DM1 at 678 nM showed less than 50% inhibition of CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, and 3A activity, indicating no reversible inhibition at the tested concentration. However, pre-incubation in human liver microsomes for 30 minutes, DM1 revealed time-dependent inhibition of CYP3A (midazolam as the substrate) (IC50 = 155 nM).
In vitro studies suggest DM1 was a substrate of P-glycoprotein (P-gp) but did not inhibit other common transporters.
#4 DM4 and DM4-Me[7]
Distribution:
DM4 and its metabolite S-methyl-DM4 (DM4-Me) exhibited very high human plasma protein binding percentages (99.38% and 99.27%, respectively). After 60 minutes of incubation, DM4’s whole blood plasma ratio decreased with increasing concentrations. While DM4-Me shows similar trends in rats and monkeys, but minimal changes in humans. Whole blood plasma ratios for DM4 and DM4-Me across species are summarized in Table 2.
Table 2. Rat, Monkey, and Human Whole Blood Plasma Ratios of DM1[7].
Compound | Rat | Monkey | Human |
DM4 | 0.907-1.62 | 0.925-4.33 | 0.488-1.85 |
DM4-Me | 0.809-1.10 | 0.635-4.04 | 0.551-0.583 |
Metabolism:
DM4 is unstable in human, rat, and monkey plasma but stable in whole blood after 90 minutes of incubation. DM4-Me remains stable in human plasma (alone or co-incubated with DM4 for 8 hours) and in human, rat, and monkey whole blood.
CYP3A4 is the primary enzyme responsible for metabolizing DM4 and DM4-Me. In cynomolgus monkeys, a single intravenous dose of 0.1 mg/kg DM4 resulted in a plasma concentration of ~0.56 µg/mL at 0.5 hours post-dose, declining below the quantitation limit (3.6 ng/mL) 7 hours post-dose, with a plasma half-life of 2 hours.
Drug Interactions:
DM4 and DM4-Me showed no induction risk for CYP1A2, CYP2B6, or CYP3A4 and no direct inhibition of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A. DM4 exhibited time-dependent inhibition of CYP3A, while DM4-Me exhibited weak time-dependent inhibition of CYP2C8 and CYP2C9. DM4 and DM4-Me were substrates of P-gp but not inhibitors.
ADC Payload Class 3: Topoisomerase Inhibitors
#5 SN-38[9]
SN-38, the active metabolite of the topoisomerase I inhibitor irinotecan, interacts with topoisomerase I to prevent religation of single-strand DNA breaks, causing DNA damage and cell apoptosis.
Distribution:
At physiological pH, the lactone ring of SN-38 can be hydrolyzed to a ring-opening form (Figure 5), and the conversion depends on the change of pH. Since only the lactone form has anti-tumor activity, minor changes in pH can affect the pharmacokinetics and efficacy of SN-38. The human plasma protein binding percentage of SN-38 is 94–96% (50–200 ng/mL). The lactone form of SN-38 dominated in plasma and was predominantly bound to albumin, and albumin also stabilized the lactone form. Its whole blood plasma ratios at concentrations of 10, 50, and 200 ng/mL were 1.46, 1.24, and 1.23, respectively [10].
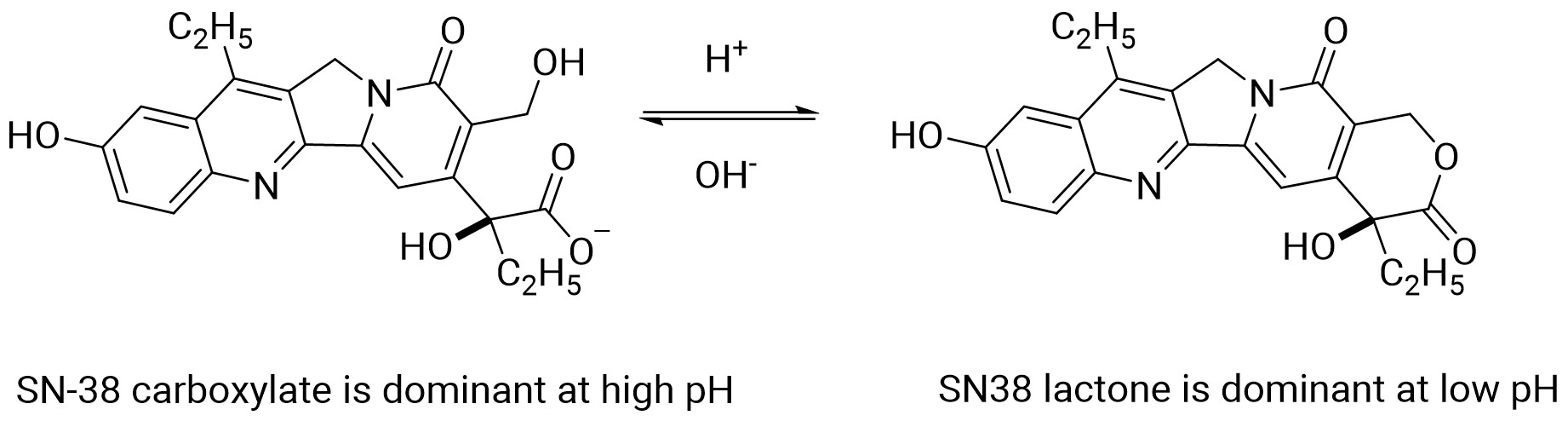
Figure 5. pH-mediated Interconversion of SN-38 Lactone and Ring-Opening Forms[9].
Metabolism and Excretion:
Uridine diphosphate glucuronosyltransferase (UGT) can mediate SN-38 glucuronidation to form the inactive SN-38G, excreted via bile. Several UGT subtypes, such as hepatic UGT1A1, UGT1A9, and extrahepatic UGT1A1, UGT1A7, UGT1A10, were involved in the transformation, among which UGT1A1, UGT1A7, and UGT1A9 are the main isoenzymes. Intestinal β-glucuronidase reactivated SN-38G to SN-38, enabling reabsorption of SN-38 in the intestine. Renal excretion contributes less to the elimination of SN-38[11].
Drug Interactions:
SN-38 was a substrate of OATP1B1.
#6 DXd
DXd, a derivative of exatecan, binds to topoisomerase I-DNA cleavable complexes, inducing double-strand DNA breaks and apoptosis of cancer cells. DXd has a TOP1 inhibitory effect similar to that of exatecan and overcomes the drug resistance of traditional microtubule-targeting agents (e.g., paclitaxel) with potent bystander effects and reduced bone marrow toxicity [1].
Distribution:
DXd exhibits moderate-to-high plasma protein binding across species (Table 3), with a human whole-blood-to-plasma ratio of 0.6.
Table 3. Plasma Protein Binding Percentages of Dxd in Mouse, Rat, Monkey, and Human (N=3) [12].
Species | Concentration (ng/mL) | Unbound (%) | Bound (%) |
Mouse | 10 | 7.5±0.6 | 92.5±0.6 |
30 | 8.5±0.6 | 91.5±0.6 | |
100 | 9.7±0.7 | 90.3±0.7 | |
Rat | 10 | 3.3±0.2 | 96.7±0.2 |
30 | 3.9±0.2 | 96.1±0.2 | |
100 | 5.8±1.4 | 94.2±1.4 | |
Cynomolgus monkey | 10 | 10.9±0.3 | 89.1±0.3 |
30 | 13.1±1.3 | 86.9±1.3 | |
100 | 13.5±0.8 | 86.5±0.8 | |
Human | 10 | 2.0±0.2 | 98.0±0.2 |
30 | 2.6±0.3 | 97.4±0.3 | |
100 | 3.2±0.1 | 96.8±0.1 |
Metabolism and Excretion:
After an intravenous injection of 1mg/kg in monkeys, DXd was rapidly cleared with a clearance of 2390 ± 499 mL/h/kg and a terminal half-life of 1.37 ± 0.766 hours (see Table 4 for PK parameters). Fecal excretion is the primary elimination route of DXd. 71.5%, 19.8%, and 1.0% of radioactive substances were found in bile, urine, and feces after an intravenous injection of 1 mg/kg [14C]DXd in bile duct-cannulated rats (total recovery: 92.3%). Most of the radioactive substances in bile are prototype [14C]DXd [13].
Table 4. PK Parameters After Intravenous Administration of Dxd in Monkeys (N=3, Mean ± Standard Deviation)
Dosage (mg/kg) | C0(ng/mL) | AUC0-inf(ng·h/mL) | T1/2 (h) | CL(mL/h/kg) | Vdss(mL/kg) |
1 | 1560 | 431±98.0 | 1.37 ± 0.766 | 2390 ± 499 | 832 ± 416 |
Drug Interactions:
In vitro assays showed that CYP3A4 mediated DXd metabolism. DXd did not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP3A, did not induce CYP1A2, CYP2B6 and CYP3A4. In vitro assays showed that DXd is the substrate of OATP1B1, OATP1B3, multidrug and toxin excretion transporter 2-K (MATE2-K), P-gp, MRP1, and BCRP. At clinically relevant concentration (steady-state Cmax~0.2 μmol/L), DXd showed low inhibitin potential for OAT1(IC50 = 12.7 μmol/L), OAT3, OCT1, OCT2, OATP1B1(IC50 = 14.4 μmol/L), OATP1B3, multidrug and toxin excretion transporter 1 (MATE1), MATE2-K, P-gp, BCRP and BSEP [12].
From the preclinical pharmacokinetic data, the drugability of DXd is not good if it is developed as a drug alone due to an excessively high clearance in cynomolgus monkeys. However, after being designed as an ADC payload, the high clearance has become an advantage and can effectively reduce the system toxicity of the payload.
ADC Payload Class 4: DNA Alkylating Agent PBD
#7 PBD
Pyrrolobenzodiazepine (PBD) binds guanine in the DNA minor groove to inhibit nucleic acid synthesis and cell division, generating cytotoxicity. The dimer form of PBD has a larger interaction area with DNA, which forms two covalent bonds with guanine to fix DNA more firmly, leading to strong cytotoxicity. The effective inhibitory concentration on various tumor cells can reach the picomolar level. Their high potency (whose IC50 could reach picomolar) positions PBDs as emerging ADC payloads, third only to auristatins and maytansinoids.
Distribution:
Human plasma protein binding percentage of PBD dimer is about 94%.
Excretion:
After intravenous injection of [3H]-PBD dimer in rats, the total radioactive substances were mainly excreted through feces and biliary tract (97.5±3.0%), and a small amount was excreted through urine (3.8±0.3%).
Drug Interactions:
At clinically relevant unconjugated concentrations, PBD dimer did not inhibit CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A. PBD dimer was a substrate for CYP3A4/5 but not for other major CYP enzymes. It is speculated that the CYP induction potential of PBD dimer is very low at clinically relevant unconjugated concentrations in serum. PBD dimer was a substrate for P-gp but not for BCRP, OATP1B1, or OCT1. At clinically relevant unconjugated concentrations, PBD dimer did not inhibit P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT2, OCT1, MATE1, MATE2-K, or BSEP [14].
ADC Payload Class 5: Soft Spongill Elebulin
#8 Eribulin [15]
Eribulin, a microtubule inhibitor, arrests the cell cycle at G2/M and disrupts mitotic spindles, leading to the termination of the mitotic process and cell death.
Distribution:
The human plasma protein binding percentage of eribulin is 49–65% at the concentration of 100–1000 ng/mL.
Metabolism and Excretion:
There are no major metabolites of eribulin in humans, and the metabolism by CYP3A4 is negligible. Following administration of 14C-eribulin, the primary circulating substance in human plasma is eribulin, with metabolite concentrations below 0.6% of the parent compound.
Eribulin is primarily excreted in its unchanged form via feces. After administration of 14C-eribulin, approximately 82% was excreted in feces and about 9% in urine, with the parent compound accounting for approximately 88% and 91% of the dose in feces and urine, respectively.
Drug Interactions:
Eribulin inhibited CYP3A activity in human liver microsomes, but it is unlikely to significantly elevate the plasma levels of CYP3A substrates. Eribulin showed no significant inhibition of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP2E1 at 5 μM. Eribulin did not exhibit induction potential for CYP1A, CYP2C9, CYP2C19, or CYP3A4 in primary human hepatocytes. At clinically relevant concentrations, eribulin may inhibit P-gp but does not inhibit BCRP, OATP1B1, OCT1, OAT1, OAT3, or MATE1. In vitro studies indicated that eribulin is a substrate for P-gp but not for BCRP, MRP2, MRP4, BSEP, OATP1B1, OATP1B3, OAT1, OAT3, OCT1, OCT2, or MATE1.
Summary of 8 ADC Payload PK Profiles The pharmacokinetic characteristics of the common 8 ADC payloads were summarized in Table 5. When the concentration of the payload in the plasma is relatively low after ADC administration, radiolabeled payloads could be recommended to be used to investigate the plasma protein binding. Enzyme phenotyping of payloads is crucial for assessing potential drug-drug interaction (DDI) risks, toxicity responses, and designing clinical combination therapies. Radiolabeled payloads would be recommended for excretion/material balance studies to ensure high recovery and clarify excretion pathways, as seen with MMAE, cys-mcMMAF, DXd, PBD, and eribulin. Evaluating interactions between payloads and transporters is essential, as they influence drug distribution and excretion in the body.
Table 5. Summary of the Pharmacokinetic Characteristics of 8 Common ADC Payloads
Payload | Distribution | Metabolism | Excretion | Drug Interactions | |
Enzyme Mediated | Transporter Mediated | ||||
MMAE | Plasma protein binding percentages in ICR mice, Wistar rats, cynomolgus monkeys, and humans are 18.8%-28.5%, 72.9-73.5%, 17.1-18.9%, and 67.9%-82.2%, respectively. | Primarily metabolized by CYP3A4; CYP2D6 may also contribute. | Excreted via bile, mainly via feces and partially via urine. | No induction potential for CYP450 enzymes; inhibits CYP3A-M with IC50 at 10 µM; no direct inhibition for CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A-T; acts as a time-dependent inhibitor of CYP3A-M and CYP3A-T. | Substrate of P-gp; not a substrate of BCRP, MRP2, OCT2, OAT1, OAT3, OATP1B1, or OATP1B3; not an inhibitor of P-gp, BCRP, BSEP, MRP2, OCT1, OCT2, OAT1, OAT3, OATP1B1, or OATP1B3. |
Cys-mcMMAF | Concentration-dependent low protein binding in human plasma: unbound percentage is 49.1-61.8% at 0.5 ng/mL, 68.8-70.7% at 5 ng/mL, and 80.9-88.7% at 50 ng/mL. | Primarily non-enzymatic with minor oxidative and Phase II metabolism. | Mostly excreted via feces; urinary excretion is the second excretion. | Not a CYP450 enzyme inhibitor or inducer. | Substrate of OATP1B1, OATP1B3, MRP1, MRP2, MRP3, and BSEP; might be a substrate of P-gp. |
DM1 | Plasma protein binding: 97.1% in rats, 91.5% in cynomolgus monkeys, and 92.5% in humans (no significant concentration dependence at 20-1000 ng/mL). | Mainly metabolized by CYP3A4 and CYP3A5. | — | No induction potential for CYP1A2, 2B6, or 3A4/5; no direct inhibition for CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, or 3A; time-dependent inhibition potential for CYP3A-M (IC50 = 155 nM). | Might be a substrate of P-gp; no inhibition potential for other common transporters. |
DM4 and DM4-Me | Very high plasma protein binding in humans: 99.38% for DM4 and 99.27% for DM4-Me. | CYP3A4 is the primary CYP450 enzyme for DM4 and DM4-Me metabolism. | — | No induction potential for CYP1A2, CYP2B6, or CYP3A4; no direct inhibition for CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A; DM4 shows time-dependent inhibition for CYP3A; DM4-Me shows weak time-dependent inhibition for CYP2C8 and CYP2C9. | Substrates of P-gp; not inhibitors of P-gp. |
SN-38 | Protein binding in human plasma: 94-96% at 50-200 ng/mL. Blood plasma ratio: 1.46, 1.24, and 1.23 at 10, 50, and 200 ng/mL, respectively. | Metabolized by UGT enzymes (UGT1A1, UGT1A7, UGT1A9 mainly). | Renal elimination contributes less to the excretion of SN-38; the inactive glucuronide metabolite of SN-38 is excreted via bile. | — | Substrate of OATP1B1. |
DXd | Moderate to high plasma protein binding in mice, rats, monkeys, and humans; blood plasma ratio in humans: 0.6. | Metabolized by CYP3A4. | Primarily excreted via feces. | No inhibition for CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A; no induction for CYP1A2, CYP2B6, or CYP3A4. | Substrate of OATP1B1, OATP1B3, MATE2-K, P-gp, MRP1, and BCRP; low inhibition potential for OAT1 (IC50 = 12.7 µmol/L), OAT3, OCT1, OCT2, OATP1B1 (IC50 = 14.4 µmol/L), OATP1B3, MATE1, MATE2-K, P-gp, BCRP, or BSEP. |
PBD Dimer | Human plasma protein binding: ~94%. | Substrate of CYP3A4/5; not a substrate of other major CYP enzymes. | Excreted via feces and bile; minor urinary excretion. | No inhibition for CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A; low induction potential for CYP enzymes at clinically relevant unconjugated serum exposures. | Substrate of P-gp; not a substrate of BCRP, OATP1B1, or OCT1; no inhibition for P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT2, OCT1, MATE1, MATE2-K, or BSEP. |
Eribulin | Human plasma protein binding: 49%-65% at 100-1000 ng/mL. | No major metabolites in humans. | Excreted primarily via feces as prototypes. | No induction for CYP1A, CYP2C9, CYP2C19, or CYP3A4; inhibits CYP3A activity but unlikely to significantly increase plasma levels of CYP3A substrates; no inhibition of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP2E1 at 5 μM in human liver microsomes. | Might be an inhibitor of P-gp; not an inhibitor of BCRP, OATP1B1, OCT1, OAT1, OAT3, or MATE1; substrate of P-gp; not a substrate of BCRP, MRP2, MRP4, BSEP, OATP1B1, OATP1B3, OAT1, OAT3, OCT1, OCT2, or MATE1. |
Conclusion and Prospect
This review summarizes the PK characteristics of ADC payloads. While traditional payloads face limitations (efficacy, resistance), novel strategies are emerging:
Photosensitizer/radionuclide-conjugates: Antibody-linked photosensitizers or radionuclides for targeted tumor killing.
siRNA conjugates: Overcoming oligonucleotide delivery barriers.
Prodrug conjugates: prodrug metabolized in the tumor to enhance specificity.
Dual-payload ADCs: Addressing resistance and tumor heterogeneity [16].
These innovations are driving next-generation ADCs with improved therapeutic windows. WuXi AppTec DMPK has established a comprehensive ADC DMPK platform and has supported hundreds of ADC projects, including dozens of IND filings.
Authors: Chong Chen, Qigan Cheng, Liping Ma, Jing Jin
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Ruan, D. Y., Wu, H. X., Meng, Q., & Xu, R. H. (2024). Development of antibody‐drug conjugates in cancer: Overview and prospects. Cancer Communications, 44(1), 3-22.
[2] http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761121s000lbl.pdf
[3]http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/125399Orig1s000PharmR.pdf
[4] Chang HP, Cheung YK, Shah DK. Whole-Body Pharmacokinetics and Physiologically Based Pharmacokinetic Model for Monomethyl Auristatin E (MMAE). J Clin Med. 2021 Mar 23; 10(6):1332.
[5]http://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761208Orig1s000MultidisciplineR.pdf
[6] Multiple discipline review of Blenrep, FDA application number 761158Orig1s000
[7]http://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761310Orig1s000MultidisciplineR.pdf
[8] http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/125427Orig1s000PharmR.pdf
[9] de Man, F. M., Goey, A. K., van Schaik, R. H., Mathijssen, R. H., & Bins, S. (2018). Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clinical pharmacokinetics, 57, 1229-1254.
[10] Combes O, Barre J, Duche JC, Vernillet L, Archimbaud Y, Marietta MP, et al. In vitro binding and partitioning of irinotecan (CPT-11) and its metabolite, SN-38, in human blood. Invest New Drugs. 2000; 18(1):1–5.
[11] http://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761115s000lbl.pdf
[12] http://www.accessdata.fda.gov/mwg-internal/de5fs23hu73ds/progress?id=0Evd2NIbMl3U-SRH8SGu4MvuaVDmrj2GBWl0LuahAKI,&dl
[13] Nagai, Y., Oitate, M., Shiozawa, H., & Ando, O. (2019). Comprehensive preclinical pharmacokinetic evaluations of trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug conjugate, in cynomolgus monkeys. Xenobiotica. Sep; 49(9):1086-1096.
[14] http://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761196Orig1s000MultidisciplineR.pdf
[15] http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/201532lbl.pdf
[16] Wang, Z., Li, H., Gou, L., Li, W., & Wang, Y. (2023). Antibody–drug conjugates: Recent advances in payloads. Acta Pharmaceutica Sinica B. Oct; 13(10):4025-4059.
Related Services and Platforms




Stay Connected
Keep up with the latest news and insights.













