Bispecific antibodies (BsAbs) are engineered antibodies that can specifically bind to two different antigens or two distinct epitopes on the same antigen. On the basis of monoclonal antibodies (mAbs), the structure of many BsAbs were modified by retaining Fc fragment, enabling Fc-mediated functions such as prolonged serum half-life, antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP). Some BsAbs were reconstituted without Fc domain, resulting in shorter half-lives, smaller molecular weights, enhanced tumor tissue penetration, and reduced immunogenicity. For example, blinatumomab has a serum half-life of 1-3 hours in animals and approximately 2 hours in humans[1]. This article summarizes the mechanism of action (MOA) of BsAbs, and pharmacokinetic (PK) characteristics and strategies of BsAbs based on regulatory submission data of marketed BsAbs[2].
Mechanism of action of bispecific antibodies
To date, 18 bispecific antibodies have been approved globally (Table 1). 15 of them target cancers, while the remaining three are used to treat hemophilia A (emicizumab), ophthalmic diseases (faricimab), and psoriasis (bimekizumab), respectively. BsAbs primarily function through three mechanisms[3]:
Cell bridging: Blinatumomab links T cells to tumor cells.
Receptor bridging: Amivantamab targets EGFR and c-Met on tumor cells, blocking multiple signaling pathways to inhibit tumor growth.
Factor bridging: Emicizumab mimics Factor VIII by bridging Factors IXa and X, promoting thrombin production.
Table 1. Marketed bispecific antibodies
Generic name (Brand name) | Targets (Mechanism) | Route of administration | Indications | Company and first approval year |
Catumaxomab (Removab) | CD3/EpCAM (T cell and tumor targeting) | IP | Malignant ascites | Trion, 2009* |
Blinatumomab (Blincyto) | CD3/CD19 (T cell and tumor targeting) | IV | Leukemia | Amgen, 2014 |
Emicizumab (Hemlibra) | FIX/FX (bridging) | SC | Hemophilia A | Roche/Chugai, 2017 |
Amivantamab (Rybrevant) | EGFR/c-Met (dual-targeting tumor) | IV | NSCLC | Johnson & Johnson, 2021 |
Bimekizumab (Bimzelx) | IL17A/IL17F (targeting IL17A and IL17F) | SC | Moderate to severe plaque psoriasis | UCB, 2021 |
Faricimab (Vabysmo) | VEGF/Ang2 (dual-targeting) | IVT | wAMD & DME | Roche, 2022 |
Candonilimab (Kaitanni) | PD-1/CTLA-4 (T cell dual-targeting) | IV | Cervical cancer | Canfite, 2022 |
Mosunetuzumab (Lunsumio) | CD3/CD20 (T cell and tumor targeting) | IV | Follicular lymphoma | Roche/Chugai, 2022 |
Glofitamab (Columvi) | CD3/CD20 (T cell and tumor targeting) | IV | DLBCL | Roche, 2022 |
Epcoritamab (Epkinly) | CD3/CD20 (T cell and tumor targeting) | IV | DLBCL | AbbVie/Genmab, 2023 |
Teclistamab (Tecvayli) | CD3/BCMA (T cell and tumor targeting) | SC | MM | Johnson & Johnson, 2023 |
Talquetamab (Talvey) | CD3/GPRC5D (T cell and tumor targeting) | SC | MM | Johnson & Johnson, 2023 |
Elranatamab (Elrexfio) | CD3/BCMA (T cell and tumor targeting) | SC | MM | Pfizer, 2023 |
Tarlatamab (Imdelltra) | CD3/DLL3 (T cell and tumor targeting) | IV | SCLC | Amgen, 2024 |
Ivonescimab (Yidafang) | PD-1/VEGF (T cell and tumor targeting) | IV | NSCLC | Akeso, Inc., 2024 |
Zanidatamab (Ziihera) | HER2(ECD4/ECD2) (tumor targeting) | IV | Biliary Tract Cancer | Jazz, 2024 |
Zenocutuzumab (Bizengri) | EGFR/HER3 (tumor targeting) | IV | NSCLC/ pancreatic adenocarcinoma | Merus, 2024 |
Odronextamab (Ordspono) | CD3/CD20 ((T cell and tumor targeting)) | IV | DLBCL/ Follicular lymphoma | Regeneron, 2024 |
*Withdrawn in 2017.
#Does not contain Fc domains
NSCLC: non-small cell lung cancer; wAMD: wet age-related macular degeneration; DME: diabetic macular edema; DLBCL: diffuse large B-cell lymphoma; MM: multiple myeloma. SCLC: small-cell lung cancer.
A review article published by YZY BioPharma in Antibody Therapeutics further categorizes the mechanisms of bispecific antibodies into six types:
Recruiting T cells to kill target cells (Figure 1B).
Dual immune checkpoint blockade. By integrating two checkpoint inhibitors into one BsAb, enhancing immune cell killing to tumor cells (Figure 1C).
Simultaneously inhibiting two signaling pathways (Figure 1D).
Co-localization blockade to inhibit two signaling pathways in tumor cells, improving anti-tumor activity than single therapies (Figure 1E).
Targeting different epitopes on the same antigen to enhance antibody interactions with tumor antigens (Figure 1F).
Tumor-targeted immune-modulating BsAb. After binding to tumor surface antigen, the BsAb modulates immune cells through another target to enhance therapeutic effects (Figure 1G).

Figure 1. Mechanism of action of bispecific antibodies[4].
Pharmacokinetic characteristics and pharmacokinetic related information of marketed BsAbs
Preclinical species selection
The selection of animal species for preclinical studies of antibodies is typically determined by pharmacodynamic (PD) experiments, which evaluate target affinity or in vitro activity. Unlike single-target monoclonal antibodies, BsAbs require species with high affinity for both targets, posing unique challenges. For example: Blinatumomab binds only to human and chimpanzee CD19/CD3, with weak or no binding to CD19/CD3 in cynomolgus monkeys, rhesus monkeys, African green monkeys, squirrel monkeys, baboons, beagles, rats, or mice. Due to its lack of affinity in rodents, a murine surrogate molecule was used for preclinical studies. The related animal species of Mosunetuzumab was cynomolgus monkey, but there was no related rodent. Instead, huCD20/CD3 transgenic mice were used to carry out related preclinical studies. Species selection aligned with ICH S6 guideline.
Dose linearity
BsAbs, like mAbs, exhibit nonlinear pharmacokinetics (PK) at lower doses due to target-mediated drug disposition (TMDD). For example, amivantamab shows linear pharmacokinetics at doses ≥20 mg/kg/week in cynomolgus monkeys, while doses <20 mg/kg/week exhibit nonlinear pharmacokinetics, possibly related to target-mediated drug disposition (TMDD). Similarly, the clearance rate of glofitamab correlates with dose and circulating B-cell counts, indicating TMDD involvement.
Gender differences
No clinically significant gender differences have been reported for marketed BsAbs.
Route of administration and absorption
Most BsAbs are administered via IV, SC, or IM; and faricimab is administered by vitreous injection (IVT) for ophthalmic diseases. The bioavailabilities of non-IV routes are generally good; for example, elranatamab has a bioavailability of 56% after SC administration, while emicizumab shows 80%-93% bioavailability with no significant differences across abdominal, upper arm, or thigh injection sites.
Distribution
None of the marketed BsAbs conducted distribution studies. Similar to monoclonal antibodies, they are generally considered to be distributed in the circulatory system and extracellular fluids due to their large molecular weights.
Elimination
BsAbs are considered to be eliminated via proteolytic degradation, like mAbs. No dedicated metabolism/excretion studies have been conducted for approved BsAbs.
Bioanalysis[5]
Ligand-binding assay platforms are primarily used in the bioanalysis of BsAbs. Given their structural complexity, four types of detection formats can be used, including total antibody, free X, free Y, and intact antibody. Ligands can serve as capture or detection reagents, and anti-idiotype antibodies may also be utilized. The choice of detection format and critical reagents should align with the target information, antibody structure, and stage of research. US Food and Drug Administration (FDA) guideline recommends using multiple detection methods to jointly characterize the structural integrity and metabolic status of BsAbs, but it is also necessary to pay attention to the bridging and interference between methods to ensure that quantitative data can refer to each other.
Notably, for specific types of BsAbs such as prodrug BsAbs which may include masking peptides, and their metabolic forms can include complete and active forms of X and Y. A variety of molecular types need to be analyzed, making ligand binding assays less suitable while LC-MS platform is more appropriate, allowing the assessment of drug integrity through the detection of surrogate peptide.

Figure 2. Detection formats for bispecific antibody bioanalysis
Immunogenicity
BsAbs’ artificial structures increase immunogenicity risks, reduce safety and efficacy, and may also lead to the formation of anti-drug antibodies (ADA) and induce serious drug-related toxicity or allergic reactions. However, clinical data of approved BsAbs indicate a low incidence of ADA. For instance, in clinical studies of emicizumab, 4 patients (approximately 2.8%) developed ADAs. In a maximum tolerated dose (MTD) study in cynomolgus monkeys, although glofitamab induced a strong ADA response (12/12), the clinical incidence was only 1.1% (5/448). In clinical trials, less than 1% of patients treated with blinatumomab developed ADAs. Mosunetuzumab’s clinical study evaluated immunogenicity in 418 patients, with no ADAs detected, possibly related to its mechanism of action, as mosunetuzumab activates T cells to kill B cells, leading to B cell depletion.
Immunogenicity studies for BsAbs should be tailored to the research phase. For early preclinical studies, analyzing total anti-drug antibodies can aid in interpreting pharmacokinetic/toxicokinetic data. During the IND stage, analyses of immunotoxicity and immune cell profiling should be included. In clinical research phases, further assessments of immunotoxicity or neutralizing antibodies (Nabs) may be necessary based on actual circumstances.
For total anti-drug antibody analysis, the classic bridging assay format is typically employed, using a three-step approach for screening, confirmation, and titer analysis.
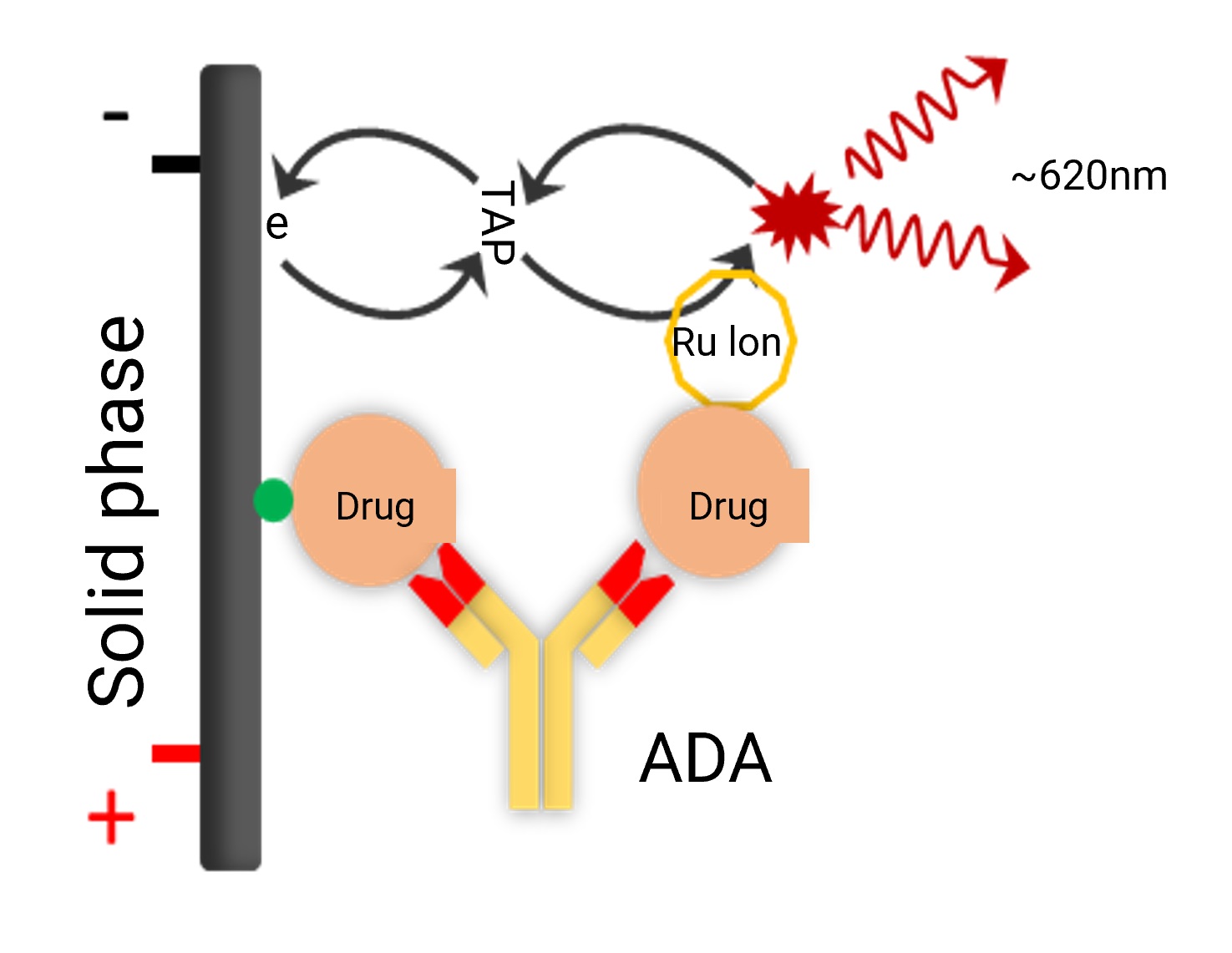
Figure 3. ADA bridging assay detection format
Cytokine release syndrome (CRS)
Most marketed BsAbs target CD3. The high affinity of CD3 can lead to T-cell activation by BsAbs even before they bind to tumor cells, triggering the rapid and severe release of cytokines such as IFN-γ, IL-6, TNFα, IL-2, IL-2R, and IL-8, which may result in a cytokine storm. Additionally, the Fc domain of bispecific antibodies may bind to Fc receptors (FcRs) on other effector cells, further increasing the risk of cytokine storms. For example, catumaxomab initiates the release of pro-inflammatory and cytotoxic cytokines upon binding to immune cells and tumor cells, with approximately 90% of patients experiencing CRS symptoms such as fever, nausea, vomiting, and chills. In clinical studies, acetaminophen was administered prior to catumaxomab to manage pain and fever. Despite preventive measures, a significant proportion of patients experienced grade 3 or higher adverse reactions, leading to catumaxomab’s withdrawal in 2017.
To mitigate CRS risk, glofitamab requires pretreatment with obinutuzumab to reduce peripheral B-cell counts before administration. However, due to potentially severe side effects, glofitamab was approved with a boxed warning. Similarly, elranatamab also poses a CRS risk, prompting clinical recommendations for stepwise dose escalation to reduce toxicity. Elranatamab, likewise, includes a black box warning. When carrying out non-clinical PK studies for CD3 targeted BsAbs, it is suggested to gradually increase the dosages. After confirming that there is no clinical side reactions for a few days at low dosage, the dosage is then increased.
Drug interactions[7]
In June 2023, the FDA issued the guidance on drug interactions (DDIs) for therapeutic proteins, pointing out that some drugs that promote the release of cytokines, such as blinatumomab, will increase the level of pro-inflammatory cytokines (such as IL-6) and down-regulate the expression of cytochrome P450(CYP450) enzyme, thus reducing the metabolism of drugs as substrates of CYP450 and increasing their exposure level.. Since most marketed BsAbs carry a risk of cytokine release, concomitant use with CYP450 substrates may require monitoring of drug concentrations and dose adjustments as needed.
The Biologics licensing application (BLA) material of Mosunetuzumab included a physiologically based pharmacokinetic (PBPK) modeling study evaluating IL-6 and CYP3A4 interactions. The results suggested a low risk of CYP450 enzyme modulation due to cytokine release caused by mosunetuzumab. The predicted area under the curve (AUC) and maximum concentration (Cmax) ratios for midazolam with versus without mosunetuzumab were 1.37 and 1.17, respectively. Thus, no dose adjustments are recommended when mosunetuzumab is coadministered with CYP3A substrates.
First-in-human dose prediction
Lessons from the clinical trial of TGN1412, a CD28-targeted T-cell agonist, is worth learning[8]. High CD3 affinity in BsAbs may overactivate T cells, potentially triggering cytokine storms. Inappropriate clinical doses could lead to severe adverse events. To ensure safety, the minimum anticipated biological effect level (MABEL) approach is recommended for the initial dose selection of CD3-targeted BsAbs. For instance, glofitamab’s first-in-human dose was determined using MABEL approach.
Final thoughts
Compared to monoclonal antibodies, BsAbs offer enhanced specificity and reduced off-target toxicity by simultaneously targeting two antigens, making them a promising frontier in antibody drug development. Emerging formats such as trispecific antibodies, multispecific antibodies, and bispecific antibody-drug conjugates are further expanding therapeutic options for patients. WuXi AppTec DMPK has supported dozens of bispecific antibody programs, leveraging extensive PK and bioanalytical expertise to accelerate the development of innovative bispecific therapies.
Authors: Qigan Cheng, Maotian Zhou, Jing Jin
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Labrijn AF, Janmaat ML, Reichert JM,et al. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov, 2019, 18, 585-608.
[2] Pharmacology review of approved bispecific antibodies.
[3] NMPA, November 2022, Technical Guidelines for Clinical Development of Bispecific Anti-tumor Drugs.
[4] Zhang J, Yi J, Zhou P. Development of bispecific antibodies in China: overview and prospects. Antib Ther. 2020, 3, 126-145.
[5] (a) Lee J W, Kelley M M, King L, et al. Bioanalytical Approaches to Quantify “Total” and “Free” Therapeutic Antibodies and Their Targets: Technical Challenges and PK/PD Applications Over the Course of Drug Development[J]. Aaps Journal, 2011, 13, 99-110. (b) FDA guidance, May 2021, Bispecific Antibody Development Programs.
[6] Gorovits B, Wakshull E, Pillutla R, Xu Y, Manning MS, Goyal J. Recommendations for the characterization of immunogenicity response to multiple domain biotherapeutics. J Immunol Methods. 2014, 408, 1-12.
[7] (a) FDA guidance, June 2023, Drug-Drug Interaction Assessment for Therapeutic Proteins Guidance for Industry. (b) Lee J, L Zhang, AY Men, LA Kenna, and SM Huang, CYP-Mediated Drug-Therapeutic Protein Interactions: Clinical Findings, Proposed Mechanisms and Regulatory Implications, Clin Pharmacokinet, 2010, 49, 295-310. [c ] Le Vee, M., Lecureur, V., Stieger, B. & Fardel, O. Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor alpha or interleukin-6. Drug Metab. Dispos. 2009, 37, 685–693.
[8] Goodyear M. Learning from the TGN1412 trial. BMJ. 2006, 332, 677-678.
[9] Trivedi A, Stienen S, Zhu M, et al. Clinical Pharmacology and Translational Aspects of Bispecific Antibodies[J]. Clinical and Translational Science, 2017, 10, 147-162.
Related Services and Platforms




Stay Connected
Keep up with the latest news and insights.













