Antibody-drug conjugates (ADCs) are innovative targeted biological drugs that link biologically active small molecules (payloads) to antibody drugs through a linker. Due to their precise targeting and effective drug delivery, ADCs represent a promising new direction in the treatment of cancer, autoimmune disease, and more. After administration, ADCs can exist in various forms within the body, including intact ADCs, biotransformation products, free payloads, linker-payload complexes, and related metabolites. Pharmacokinetic studies of ADCs typically require the assessment of total antibodies, conjugated antibodies, free payloads, drug-to-antibody ratios (DAR), conjugated payloads, drug linkers, relevant metabolites, and the immunogenicity of the ADC. A variety of bioanalytical platforms are needed to understand the actual role of ADCs in disease, including ELISA, LC-HRMS, and LC-MS/MS. Current focus areas include the in vivo changes of total antibodies, conjugated antibodies, conjugated payloads, and free drugs.
In recent years, due to the development of liquid chromatography-mass spectrometry (LC-MS) technology for protein detection, as well as the optimization of enzymes used to process proteins, the ability to use mass spectrometry platforms to detect ADC total antibodies and conjugated payloads in biological samples has developed rapidly. In this article, we use Enhertu (DS-8201) as an example to summarize an analytical method that can simultaneously detect the total antibody and conjugated payload of an ADC drug in a single sample.
LC-MS platform for detecting total antibodies and conjugated payloads
In biological analyses of in vivo samples, particularly in experimental models like mice, sample availability is often limited. ADC-related analyses typically involve both small and large molecular components, increasing the demand for sample volume, especially when assessing immunogenicity. Additionally, the immunocapture process for large molecules is time-consuming and reagent-intensive. If both total antibodies and conjugated payloads can be assessed from the same sample, it would reduce overall sample requirements and streamline processing.
When detecting total antibodies using the LC-MS platform, the antibodies are usually enriched and purified before being enzymatically digested. Characteristic peptide segments are then selected for quantification to determine total antibody concentration. For detecting conjugated payloads, appropriate dissociation methods—such as enzymatic digestion (e.g., trypsin, papain) or pH adjustment—are employed to release the payload for analysis. To achieve lower detection limits, optimization is needed across several factors, including peptide selection, ADC immuno-enrichment, enzyme choice, digestion conditions, and LC-MS settings.
Case study: DS-8201
DS-8201 is an antibody conjugate that links a monoclonal antibody targeting HER2 with a cleavable linker and a topoisomerase I inhibitor. It has been approved for treating metastatic HER2-positive breast cancer patients. DS-8201 effectively delivers the small molecule payload to cancer cells, even in cases of low HER2 expression, addressing the limitations of earlier ADCs. We present a method for the simultaneous detection of total antibodies and conjugated payloads in the same sample using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS).
The sample preparation workflow for DS-8201 is illustrated in Figure 1.
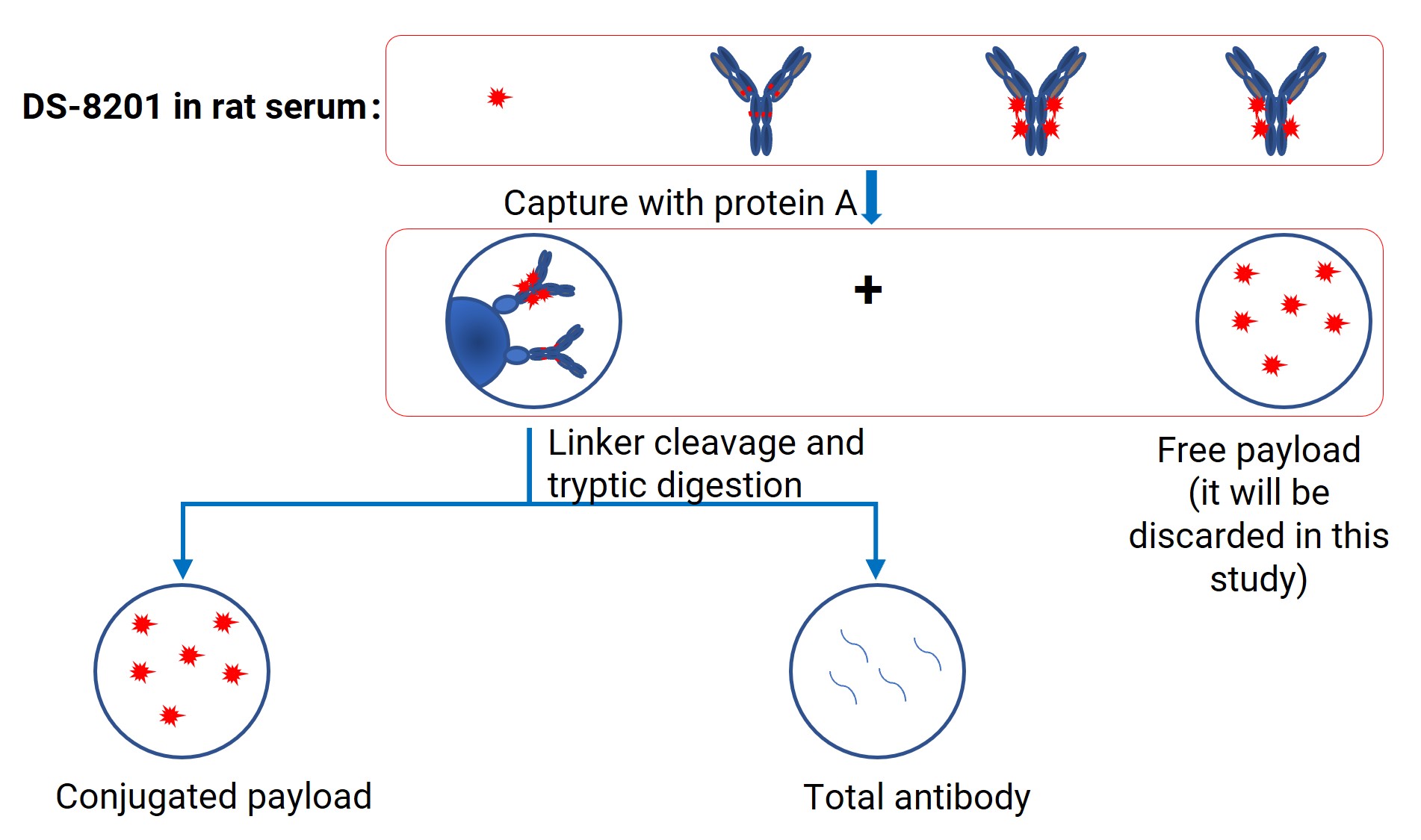
Figure 1. Sample processing workflow for DS-8201 in the biological matrices

Figure 2. DS-8201 structure
The specific operational steps include:
#1 Mass spectrometry section: identifying surrogate peptides and tuning the MS.
-
Screening suitable ion pairs for the small molecule payload Dxd.
-
Selecting unique or generic surrogate peptides from the antibody part and their optimal multiple reaction monitoring (MRM) ion pairs.
-
Using software to predict the unique or generic surrogate peptides and corresponding ion pair information based on the amino acid sequences of DS-8201 and the internal standard protein.
-
After digesting DS-8201 and the internal standard protein with trypsin, surrogate peptides, and corresponding ion pairs, are identified using mass spectrometry, with software analysis confirming the results.
#2 Liquid chromatography section: optimizing the liquid chromatography gradient and sample preparation.
-
Dual enzymatic digestion using both trypsin and papain for serum samples of DS-8201 captured by protein A.
-
Adjusting the liquid chromatography gradient based on digestion results to obtain the optimal chromatographic method.
-
The linear range for total antibodies and conjugated payloads in SD rat serum is 2–600 nM, while linearity, sensitivity, accuracy, precision, matrix effects, recovery, and selectivity are assessed within this range.
Results and discussions
Following the above procedure, we developed an RPLC-MS/MS method for the simultaneous detection of total DS-8201 antibody and conjugated payload in the serum of SD rats with a separation time of only 8 minutes. DS-8201 was used to prepare a standard curve of 2-600 nM, and the fitting results of the standard curve are shown in Table 1.
|
Item |
Cursing range |
Fit weights |
r2 |
|
Total antibodies |
2 ~ 600 nM |
1/X2 |
0.9962 |
|
Conjugated load: DXD |
2 ~ 600 nM |
1/X2 |
0.9972 |
Table 1. The linear range of DS-8201 total antibody and conjugated payload in SD rat serum
After purification and extraction, DS-8201 in SD rat serum was analyzed for total antibodies and conjugated payloads using trypsin and papain digestion methods. The digestion efficiency, matrix effects, recovery, and selectivity of each method within the 2–600 nM range were evaluated.
By comparing the trypsin digestion efficiency of trastuzumab and DS-8201, it can be seen that the digestion efficiency of this analytical method reached 89%~100%, which met the analysis requirements.
Additionally, the efficiency of papain in hydrolyzing the peptide segments post-trypsin digestion to release Dxd was also assessed (Figure 3). Results indicated that papain’s hydrolysis efficiency ranged from 92% to 98%, also satisfying analytical needs.
After optimizing the liquid chromatography conditions, the residues of the characteristic peptides and Dxd we selected were smaller than the LLOQ, which met the analytical requirements, and the chromatogram of the LLOQ is shown in Figure 3.
 Figure 3. Chromatogram of LLOQ sample of DS-8201 in rat serum after papain and trypsin digestion: total antibody (A) and conjugated payload (B)
Figure 3. Chromatogram of LLOQ sample of DS-8201 in rat serum after papain and trypsin digestion: total antibody (A) and conjugated payload (B)
Since the concentration of compounds in samples can vary over time, it is essential to assess whether the detected concentrations of total antibodies and conjugated payloads align with expectations following sample dilution. Our evaluations showed that the dilution consistency deviation for both total antibodies and conjugated payloads was less than 15%, which meets the analysis requirements.
Bioanalytical strategy for ADCs using LC-MS platforms
The LC-MS platform can analyze various ADC-related research subjects, including total antibodies, conjugated payloads, conjugated antibodies, free payloads, total payloads, naked antibodies, DAR values, and biotransformation products. To differentiate these subjects, careful selection of reagents for immunocapture, enzymes for digestion, and appropriate LC-MS platforms is crucial, as illustrated in Figure 4.
For total antibodies, conjugated payloads, and ADC biotransformation, a common approach is to immunocapture all ADC-related macromolecules from biological matrices before further processing. Depending on ADC concentration, biological matrix, and specificity requirements, various capture agents such as protein A/G, anti-human antibodies, unique-type antibodies, or target proteins may be employed.
If the LC-MS platform aims to detect conjugated antibodies, generic immunocapture reagents can be used to identify ADCs with different DAR values through LC-HRMS. By utilizing capture antibodies specific to the payload and employing characteristic peptide methods for quantification, better sensitivity can be achieved in the detection of conjugated antibodies.
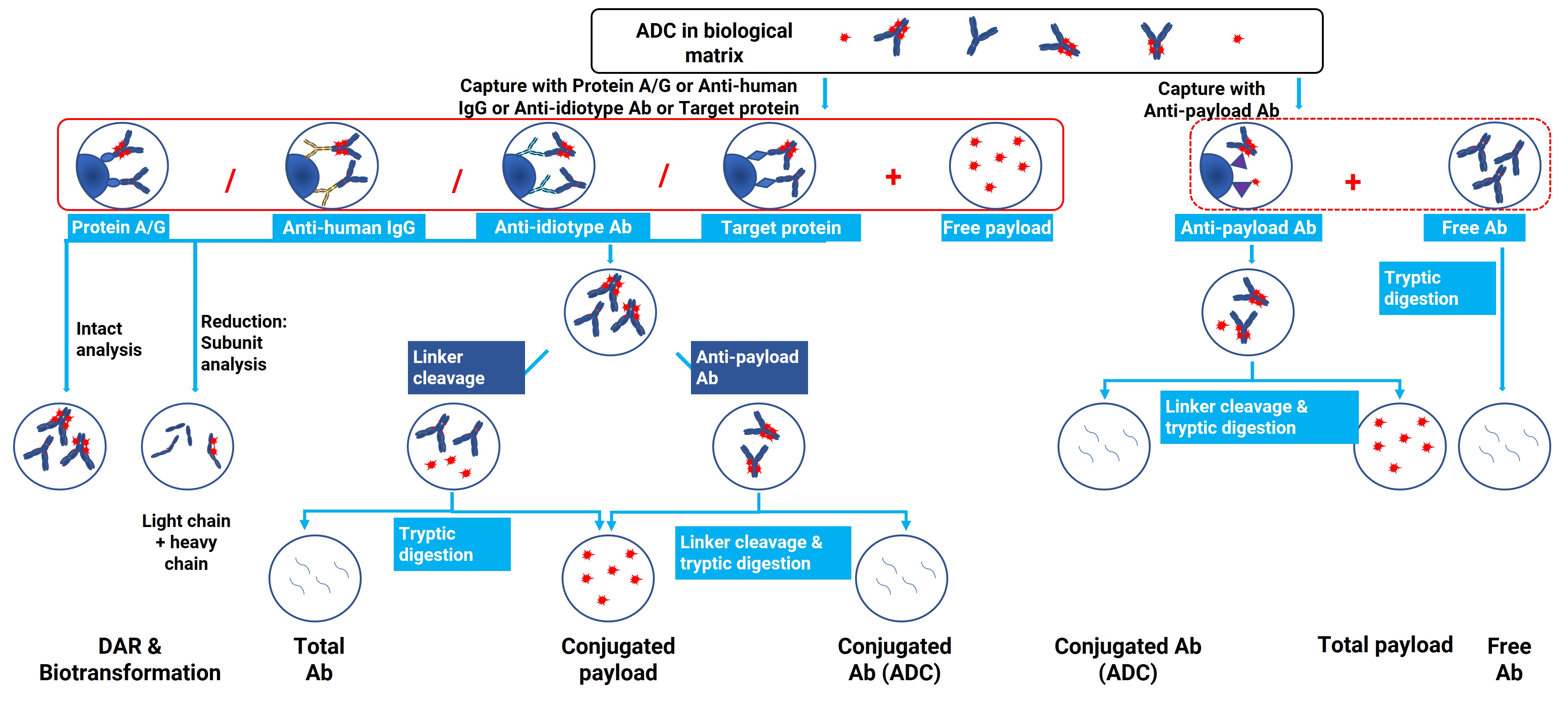
Figure 4. Bioanalytical strategy tree of ADC-related research subjects
Using mass spectrometry to detect total antibodies and conjugated payloads in ADCs enhances the capability for preclinical sample analysis. ELISA methods cannot be employed for detecting conjugated antibodies without corresponding anti-payload antibodies. However, through immuno-enrichment and enzymatic hydrolysis of samples, LC-MS/MS can accurately detect characteristic peptides and small molecule payloads. This facilitates the detection of both conjugated payloads and total antibodies, reducing the reliance on specific antibodies and reagents. It also enables average DAR value detection, providing researchers with alternative methods to assess ADC DAR values beyond high-resolution mass spectrometry.
Conclusion
This article illustrates the development of an LC-MS/MS method for detecting total antibodies and conjugated payloads in rat serum using DS-8201 as an example. By employing similar sample processing steps, multiple parameters can be assessed on the same detection platform. This significantly simplifies ADC detection in animal samples. Method optimization has achieved detection limits of 2 nM for both parameters, meeting pharmacokinetic research requirements.
WuXi AppTec DMPK has established a testing platform for ADCs to evaluate their behavior in animals. Equipped with LC-MS/MS, LC-HRMS, ELISA, MSD, flow cytometry, and quantitative whole-body autoradiography (QWBA), this platform meets diverse analytical needs and provides integrated services to accelerate new drug development.
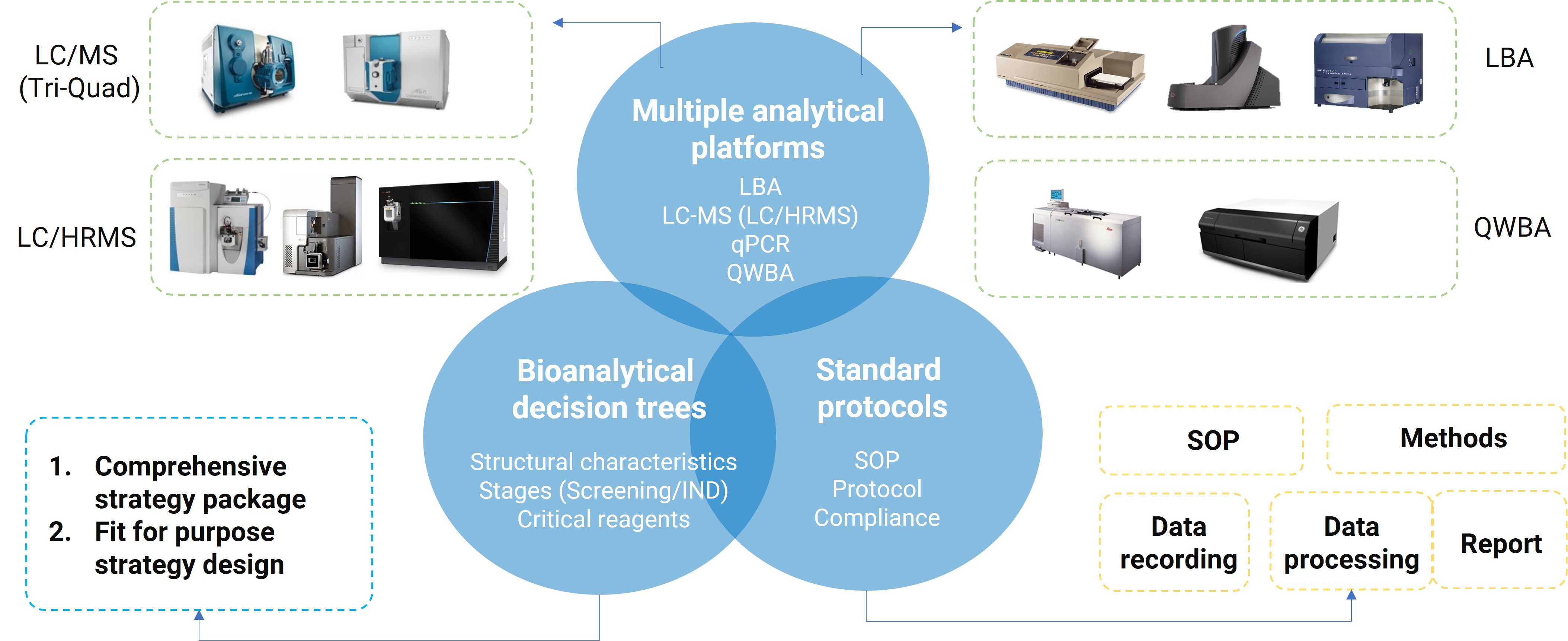
Figure 5. A comprehensive DMPK bioanalytical platform
Authors: Zhiren Yu, Hefeng Zhang, Wenhan Zhang, Zhiyu Li, Hongmei Wang, Lili Xing
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Preeti Narayan , Christy L Osgood, Harpreet Singh, Haw-Jyh Chiu, Tiffany K Ricks, Edwin Chiu Yuen Chow, Junshan Qiu, Pengfei Song, Jingyu Yu, Frances Namuswe, Maria Guiterrez-Lugo, Sherry Hou, William F Pierce, Kirsten B Goldberg, Shenghui Tang , Laleh Amiri-Kordestani, Marc R Theoret, Richard Pazdur , Julia A Beaver; FDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki for the Treatment of Unresectable or Metastatic HER2-Positive Breast Cancer;Clin Cancer Res. 2021 Aug 15; 27(16): 4478–4485.
Related Services and Platforms




-

 DMPK BioanalysisLearn More
DMPK BioanalysisLearn More -

 Novel Drug Modalities DMPK Enabling PlatformsLearn More
Novel Drug Modalities DMPK Enabling PlatformsLearn More -

 Novel Drug Modalities BioanalysisLearn More
Novel Drug Modalities BioanalysisLearn More -

 Small Molecules BioanalysisLearn More
Small Molecules BioanalysisLearn More -

 Bioanalytical Instrument PlatformLearn More
Bioanalytical Instrument PlatformLearn More -

 PROTAC DMPK ServicesLearn More
PROTAC DMPK ServicesLearn More -

 ADC DMPK ServicesLearn More
ADC DMPK ServicesLearn More -

 Oligo DMPK ServicesLearn More
Oligo DMPK ServicesLearn More -

 PDC DMPK ServicesLearn More
PDC DMPK ServicesLearn More -

 Peptide DMPK ServicesLearn More
Peptide DMPK ServicesLearn More -

 mRNA DMPK ServicesLearn More
mRNA DMPK ServicesLearn More -

 Covalent Drugs DMPK ServicesLearn More
Covalent Drugs DMPK ServicesLearn More
Stay Connected
Keep up with the latest news and insights.
















