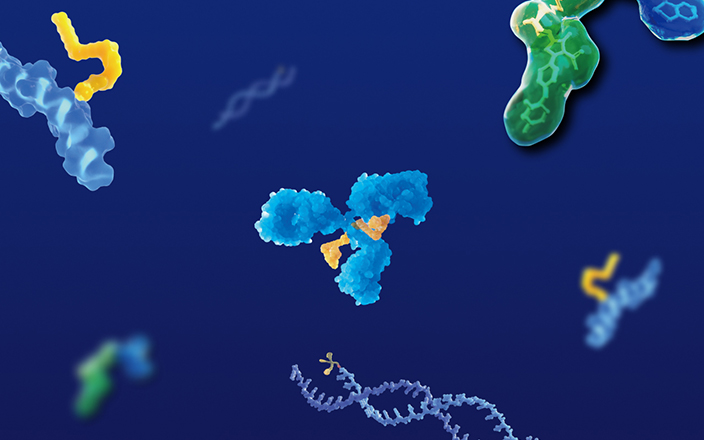
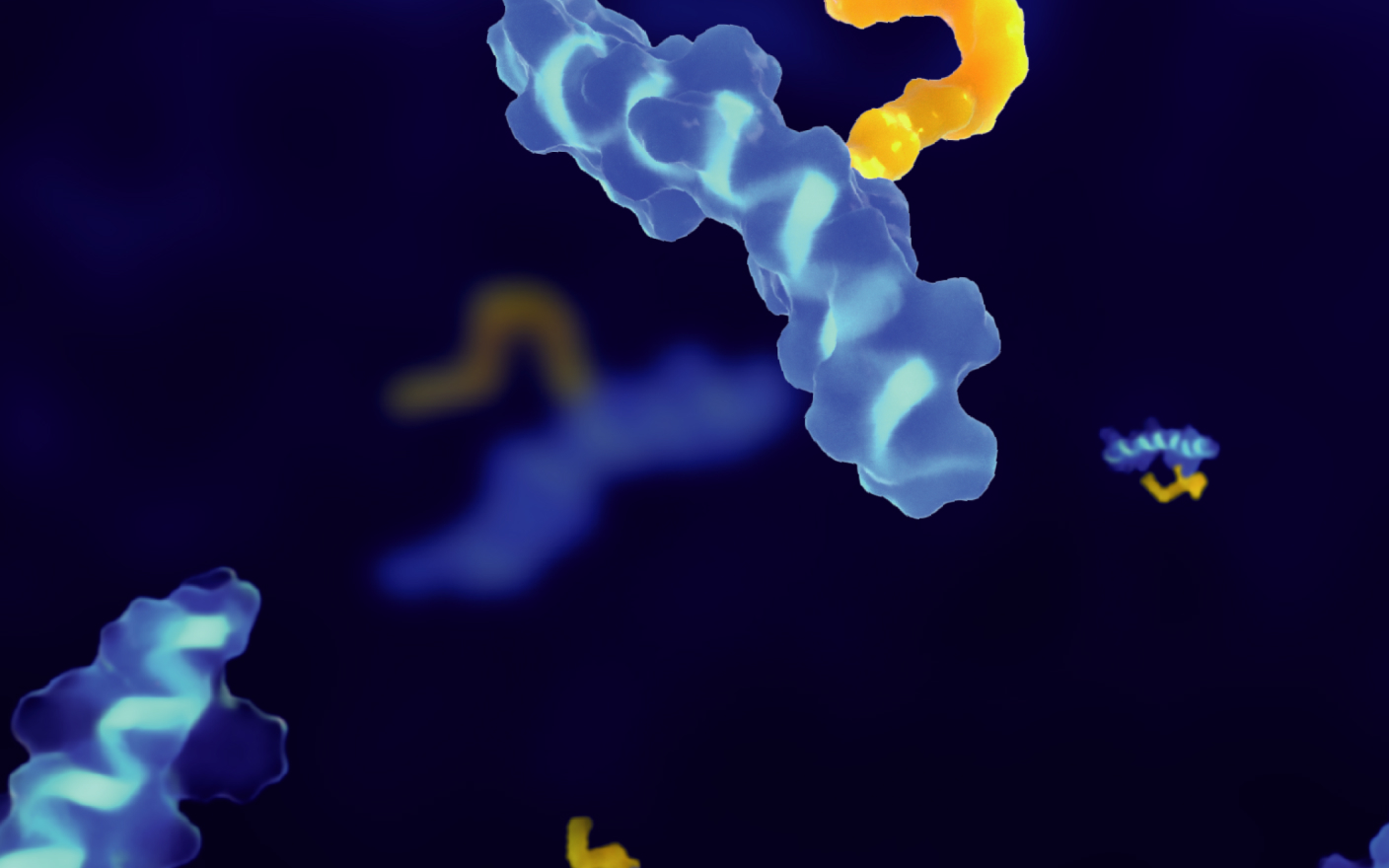
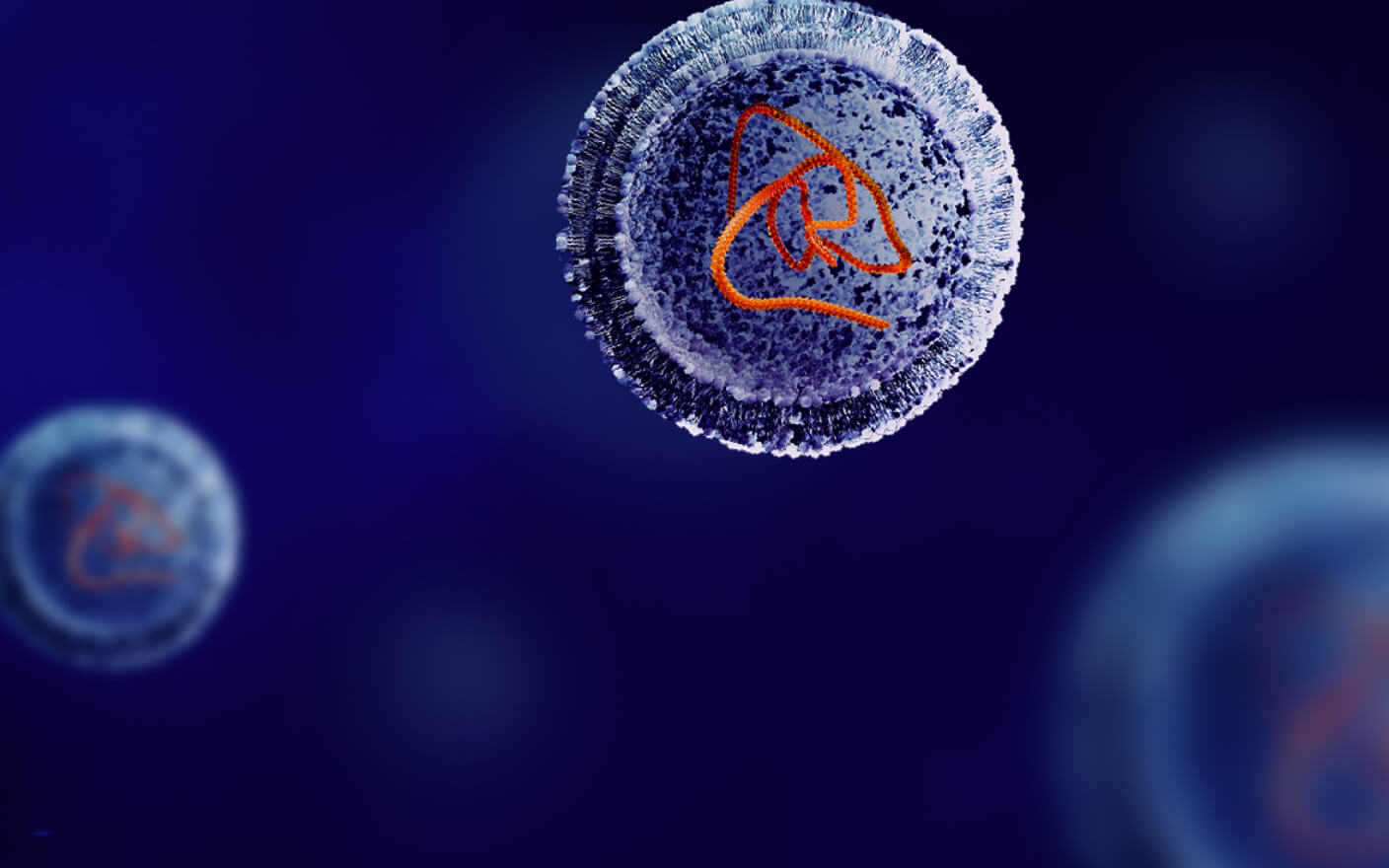
Antibody-drug conjugates (ADC), as a novel antitumor therapeutic drug, are formed by the conjugation of monoclonal antibodies with highly toxic payloads through linkers. By the end of 2022, a total of 15 ADCs have been approved globally, among them 10 ADCs were approved since 2019, triggering a new boom in ADC development. ADC consists of three components: the antibody, the toxic payload, and the linker connecting the antibody with the payload. There are two key factors for the successful development of novel ADCs: First, an ADC should remain stable in blood circulation and attack tumor cells selectively, leading to the rapid release of the ADC payload after being endocytosed by tumor cells. Poor stability in blood circulation or excessive distribution in other tissues can result in severe toxicity of ADCs. Second, the payload released by an ADC in tumor cells should be potently cytotoxic to kill tumor cells quickly (Figure 1).

Figure 1. ADC structure
ADC PK evaluation
From a pharmacokinetic perspective, ADC development exhibits two characteristics. First of all, the ADC PK study is challenging. As a novel drug, there is limited understanding of ADCs’ human PK properties within the industry. To date, no human radiolabeled mass balance studies have been conducted for ADCs. ADC is a combination of large and small molecules, making it necessary to study the pharmacokinetics of these two molecules. Secondly, the study of the ADC payload release and metabolism is essential for ADC development. Specifically, the release and concentration of payloads in target organs are directly related to ADC efficacy, while their release in non-target tissues is directly related to ADC toxicity. Additionally, the Drug-Drug Interaction (DDI) of ADC is also associated with exposure, metabolism, and excretion of payload (Figure 2).

Figure 2. Challenges and importance of ADC drug study
To date, the most authoritative document in the literature concerning the ADC PK study is “Current Approaches for Absorption, Distribution, Metabolism, and Excretion Characterization of Antibody-Drug Conjugates: An Industry White Paper” published by the American Society for Pharmacology and Experimental Therapeutics in 2016. However, the PK studies proposed in the white paper are relatively basic and include only human and animal PK studies after ADC administration, animal ADME studies, and the identification of small molecule metabolites in humans and animals. Additionally, the white paper showed that PK studies of payload should be carried out directly, including animal PK studies, in vitro metabolite identification, and DDI studies. However, this white paper lacks detailed descriptions of the strategies and the methods and scope of ADC PK studies have been evolving in recent years. Therefore, ADC PK studies should be tailored.
In July 2022, the Chinese National Medical Products Administration (NMPA) publicly solicited comments on the "Guidelines for Non-clinical Studies of Antibody-Drug Conjugates (Draft for Comment)" 1. In this draft guidelines, the preclinical pharmacokinetics of ADC drugs can be mainly divided into three parts as shown in the table below, the corresponding research on ADC, new free small molecule compounds, and new linkers. Free small molecule compounds refer to free payload and other free payload-related components released from ADC. To know which new free small molecule compounds need to be evaluated, payload-related metabolite profiling and identification study for ADC need to be conducted.
|
Testing article |
Study contents |
|
ADC |
In-vitro stability |
|
Plasma concentration-time curve |
|
|
Tissue distribution of ADCs intended to target specific tissue |
|
|
New free small molecule compounds |
Plasma protein binding |
|
Absorption, distribution, metabolism, and excretion |
|
|
Drug-drug interaction with drug metabolism enzymes and drug transporters |
|
|
New linkers |
In-vivo metabolism |
Table 1. Preclinical ADC PK studies
ADC PK study strategies
In recent years, ADC development projects in the Chinese pharmaceutical industry have flourished. WuXi AppTec DMPK Service Department has specially established a team devoted to developing ADCs and they have studied and summarized the PK studies and methodologies of FDA-approved ADCs.
The ADC PK studies have been categorized into four main aspects: ADME, in vitro DDI, PK, and bioanalysis. Each of these aspects holds particular significance in drug discovery, pre-clinical development, and clinical stages (Table 2). WuXi AppTec DMPK will propose a specific PK study strategy and specific study protocols for each ADC project based on the structure of payloads to accelerate drug development and registration.

Table 2. Pharmacokinetic studies of ADCs at different stages of research
Based on the literature, analysis of pharmacokinetic data from approved ADC drugs, and summarizing of research experience, we have identified four specific requirements for ADC PK research:
-
It requires the ability to conduct PK studies of small and large molecules.
-
It requires comprehensive capabilities and facilities for ADME and bioanalysis, including study capacity for small and large animals, bioanalysis of large and small molecules, in vitro DDI, in vitro and in vivo metabolite identification, and radiolabeled ADME.
-
It is necessary to specifically design the overall strategies for PK studies and the specific protocols according to the structure of the ADC and the demand for the effectiveness and safety of new drugs.
-
The ADC PK study requires excellent project management capability as well as the ability to coordinate and collaborate across multiple departments
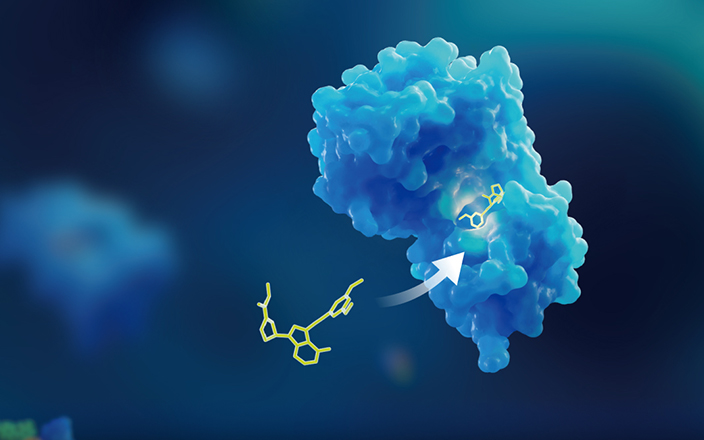
The Relationship between ADC payload release and ADC Toxicity as well as DDI
It is worth noting that the release, metabolism, and clearance of ADC payloads in non-target tissues are closely related to ADC toxicity and DDI. ADC can be divided into two types based on their structure: One is cleavable ADCs that release payloads under the catalysis of lysosomal enzymes or acidic conditions, and the released payloads have a relatively clear structure. The other is non-cleavable ADCs that release payloads that are bound to partial peptide chains or amino acids through the degradation of antibodies and the structures of payload-related metabolites are unknown. The exposure of the payloads released from ADCs to non-target tissues may be toxic. If the metabolism or clearance of these ADC payloads is affected by metabolic enzyme inhibitors, their systemic exposure will increase and thus result in toxic effects (Figure 3).

Figure 3. Payloads released from ADCs
To support our clients’ ADC projects, the WuXi AppTec DMPK ADC team has specifically developed strategies, processes, and methods for the study of the release and metabolism of ADC payloads. For cleavable ADCs, the in vitro metabolic system of ADCs is used to determine whether payloads are released according to the ADC design. For non-cleavable ADCs, the same metabolic system is used to identify payload-related metabolites through proteolysis.
Once the identification of released small molecules is completed, if there is previously studied PK data of these small molecule compounds, ADME and DDI studies for the payloads are unnecessary. However, a new payload must conduct a series of studies. Such studies mainly focus on two aspects. The first is the metabolite identification of ADC payloads, including the comparison of in vitro metabolites in multiple species, animal ADME studies, and small molecule metabolites identification in human plasma; the second is the in vitro DDI studies, including CYP450 enzyme induction and inhibition, metabolic enzyme phenotyping, inhibition or substrate evaluation of drug transporters. The results of these studies provide a scientific basis for the development and registration of ADCs, such as guidance for the selection of toxic species, genetic polymorphism study of metabolic enzymes, clinical DDI study design, and studies for special populations.
Click here to learn more about the strategies for ADC, or talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Authors: Liping Ma, Jie Wang, Nan Zhao, Ruixing Li, Mingshe Zhu
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,500+ global clients, and have successfully supported 1,200+ IND applications.
Reference
[1] the National Medical Products Administration (NMPA) publicly solicited comments on the " Guidelines for Non-clinical Studies of Antibody-Drug Conjugates (Draft for Comment)”.2022
Related Services and Platforms




Stay Connected
Keep up with the latest news and insights.